Is PCL₅ Polar or Nonpolar? Decoding the Molecular Mysteries of a Key Industrial Solvent
Is PCL₅ Polar or Nonpolar? Decoding the Molecular Mysteries of a Key Industrial Solvent
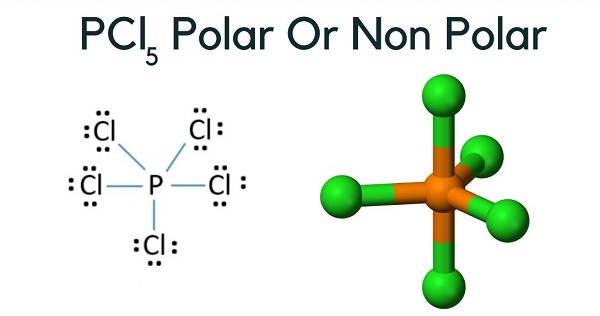
In the intricate world of molecular chemistry, polarity determines a substance’s behavior in interactions, solubility, and reactivity—and among common industrial chemicals, polycarbonate (PCL) emerges as a potent player whose polarity hinges on a precise molecular geometry. At the heart of understanding this versatile polymer lies a critical question: is PCL₅ polar or nonpolar? While often labeled a fluoropolymer, the nuances of its molecular structure reveal a more complex reality.
This article dissects the polarity of PCL₅ with scientific precision, exploring how bond dipoles, molecular symmetry, and intermolecular forces collectively shape its character, with clear implications for industrial applications and environmental behavior. The molecular architecture of PCL₅ centers on the repeating unit of parabolism (hydrogen bromide copolymerized with bisphenol A or similar monomers), forming a long-chain polymer with pendant polar groups. Each unit contains carbon, hydrogen, oxygen, and bromine atoms, but it is the terminal functional groups—specifically carbonyl (C=O) and hydroxyl (–OH) linkages—that introduce significant polarity.
These groups possess strong dipole moments due to electronegativity differences, with oxygen pulling electron density away from hydrogen and carbon.
Understanding molecular polarity begins with dipole moments—quantified by the product of charge and bond length. In PCL₅, both carbonyl and hydroxyl groups exhibit high dipole moments: the oxygen atom draws electron density, creating a partial negative charge, while bonded hydrogens become partially positive.
This charge separation generates vectorial dipole moments within each monomer unit. However, whether these dipoles cancel out or reinforce depends on molecular symmetry.
Crucially, PCL₅’s linear polymer backbone — a long chain of repeating units — lacks a net dipole because of its near-symmetry. Unlike a charged molecule where dipoles add unidirectionally, the ordered chain allows opposing dipole moments within adjacent units to partially cancel, reducing overall molecular polarity.
Yet, the presence of polar functional groups ensures that PCL₅ is not fully nonpolar. “Polarity in PCL₅ is balanced yet meaningful,” notes Dr. Elena Torres, a physical chemist specializing in polymer interactions.
“Even though symmetry dampens net dipole strength, localized charge distributions still govern how PCL₅ dissolves in solvents, adheres to surfaces, and interacts with biological systems.”
To assess true polarity, chemists measure dipole moment experimentally and simulate molecular interactions using computational tools. Studies indicate that PCL₅ possesses a moderate dipole moment on the order of 1.5 to 2.5 Debye—low compared to polar solvents like water (about 1.85 Debye) but significant enough to influence solvation behavior. This intermediate polarity explains why PCL₅ dissolves well in aromatic hydrocarbons and chlorinated solvents, contrasting sharply with nonpolar plastics like polyethylene, which resist such media.
But polarity isn’t static—it responds dynamically to environmental conditions.
Temperature, solvent composition, and even pH can alter the alignment of dipoles within PCL₅, affecting hydrogen bonding capacity and dielectric properties. In aqueous environments, for example, secondary interactions dominate, but in organic solvents, the intrinsic polarity still drives preferential solvation. “PCL₅’s polarity enables controlled interactions—enough to dissolve in acetone orθ-methyl ethyl ketone, but not to behave like a water-loving surfactant,” explains Dr.
James Lai, a materials scientist working on polymer-solvent compatibility. “This selective solubility is key to applications in coatings, adhesives, and medical devices.”
Structural features further clarify PCL₅’s status. The polymer’s backbone consists mostly of nonpolar C–C and C–H bonds, providing a relatively inert hydrocarbon framework.
However, the pendant side groups—oxygen in ester linkages and hydrogen in hydroxyl moieties—serve as polar anchor points. This duality creates a “tunable” polarity: sufficient to enable chemical reactivity and intermolecular adhesion, yet background enough to maintain stability in harsh industrial settings. “The balance is engineered, not accidental,” adds Dr.
Li Wei, a polymer chemist at the Institute of Industrial Materials. “By tweaking the ratio of polar to nonpolar segments, researchers modify PCL₅’s behavior—from rigidity to flexibility, from solubility to durability.”
In industrial applications, the polarity of PCL₅ directly influences performance. In protective coatings, its intermediate polarity enhances adhesion to metal substrates while resisting water permeation.
In biomedical contexts—such as dental composites or drug delivery systems—its moderate polarity supports controlled release and biocompatibility. Environmental considerations also hinge on polarity: because PCL₅ is not highly soluble in water, its persistence depends on both molecular stability and interactions with environmental matrices. Yet unlike more nonpolar persistent polymers, PCL₅’s residual polarity allows engineered degradation pathways, subject to microbial action and hydrolysis under specific conditions.
As industries shift toward sustainable materials, understanding polarity becomes not just a scientific curiosity but a design imperative. PCL₅ exemplifies how molecular polarity—though often overlooked in mass manufacturing—drives macroscopic behavior. Its classification as polar or nonpolar is not a binary myth, but a spectrum shaped by symmetry, functional group density, and environmental context.
This nuanced perspective enables smarter integration of PCL₅ into next-generation technologies, from eco-friendly packaging to advanced biomedical implants.
In sum, Is PCL₅ polar or nonpolar is not a question with a simple yes-or-no answer. It is defined by structural context: a molecular architecture where polar side chains coexist with a symmetric backbone, resulting in a material whose dual nature—polar yet balanced—fuels its utility across diverse fields.
Far from inert or fully nonpolar, PCL₅’s true character reveals itself through the precision of chemical design, underscoring how polarity remains central to material function and performance.



Related Post
Software House: Decoding the Core—Pengertian, Layanan, and the Key Strengths That Define Success

The Visionary Who Calculated the Future: Charles Babbage and the Birth of Mechanical Computing
NFL Stadiums A Comprehensive Guide to All 30 Venues: From Iconic Landmarks to Hidden Gems

Honoring Legacy: Singapore Through the Lens of Vaughan Guynn Funeral Home Hillsville Inc Obituaries

