What Is The Charge Of A Single Electron? The Atomic Harbor of Electrical Currency
What Is The Charge Of A Single Electron? The Atomic Harbor of Electrical Currency
A single electron carries a fundamental electrical charge that defines the very behavior of matter at the quantum level. This charge, quantified with remarkable precision, stands as one of the most precise constants in physics—an invisible currency flowing through atoms, enabling everything from electrical circuits to chemical reactions. With a magnitude of exact value, the charge of a single electron is −1.602176634 times 10⁻¹⁹ coulombs, a figure so exact it serves as a cornerstone for electromagnetic theory and modern technology alike.
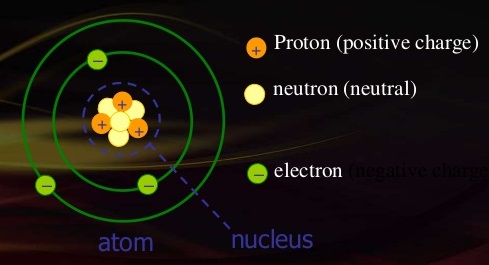
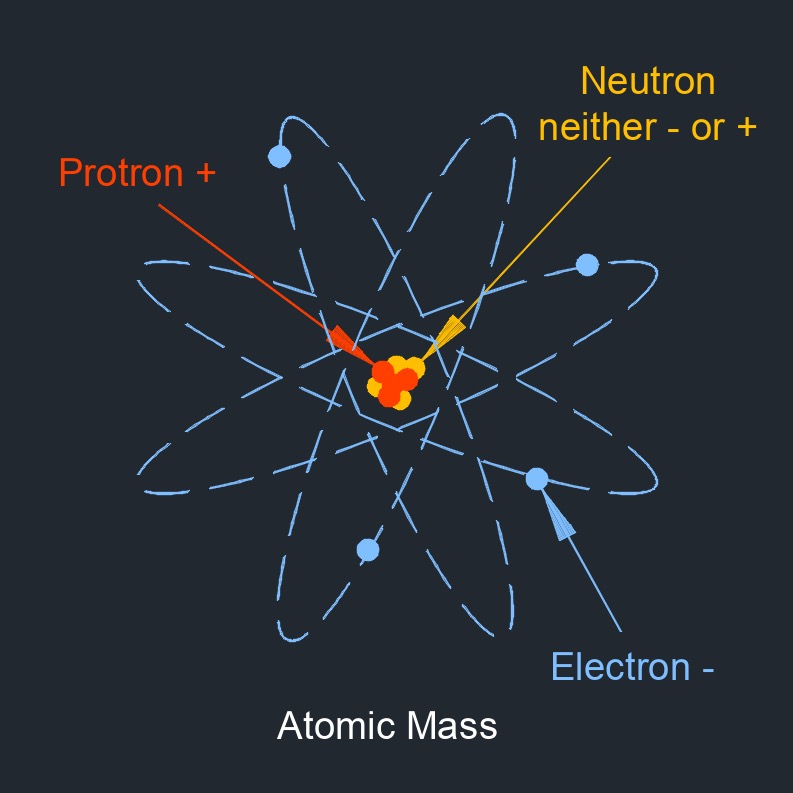
Definition and Magnitude of Electron Charge At the heart of atomic structure lies the electron, a negatively charged subatomic particle. In the International System of Units (SI), the unit of electric charge is the coulomb, but due to the electron’s extremely small magnitude, the value is expressed in excitocurrents (includes the coulomb, but used commonly for electron-scale charge). The accepted precise value of the elementary charge—defined as the charge of a single electron—is exactly −1.602176634×10⁻¹⁹ coulombs.
This value was established through refined experiments, including the iconic Millikan oil-drop experiment, which first measured the charge of electrons with unprecedented accuracy. Historical Context: Measuring the Infinitesimal Charge The journey to quantify the electron’s charge began in the early 20th century. Before this discovery, electricity was understood as a flow of “quantity,” but without a fixed unit tied to a physical particle, theory remained largely qualitative.
In 1909, Robert A. Millikan’s oil-drop experiment dramatically changed this by isolating individual charged droplets and measuring their motion under electric forces. By balancing gravitational, buoyant, and electrostatic forces, Millikan determined the discrete charge value of the electron.
His work revealed charge is quantized—a key insight that electrons are not infinitely divisible but carry exact multiples of this fundamental value. > “The electron’s charge is not approximate in principle; it is known to better than one part in a billion,” notes physicist

Related Post

Aayushi Jaiswal Web Series List: Your Essential A-to-Z Guide to India’s Rising Star Actress
Argument Made for Why Mercedes Mone Joining AEW Wont Boost Business
Costa Mesa Police Response Times: Behind the Numbers, Thousands Wait Too Long for Help

Warsaw America: How Poland’s Rising Influence Reshapes Washington’s Global Strategy