What Is the Center of an Atom? Unveiling the Atomic Nucleus
What Is the Center of an Atom? Unveiling the Atomic Nucleus
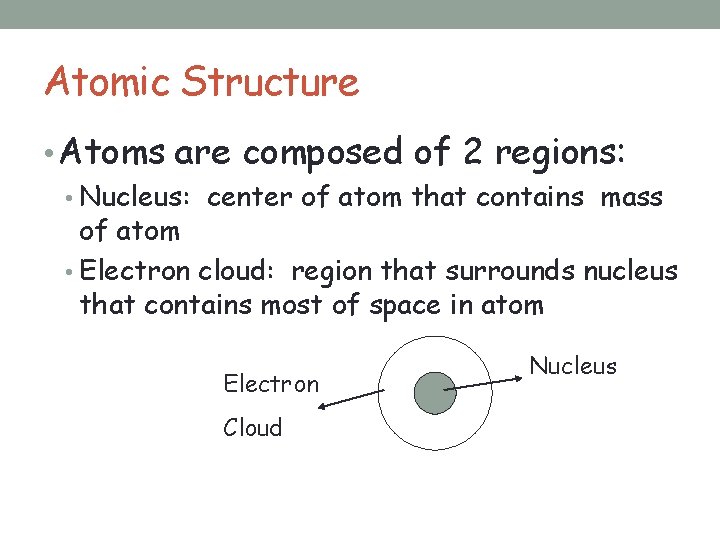
Below the surface of all matter lies a hidden world governed by principles precision and mystery. At the heart of every atom lies a dense region known as the nucleus—the central core where the atom’s mass is concentrated. With a diameter measuring just about 10⁻¹⁵ meters, the nucleus is an extraordinary zone of immense power within an otherwise electron-dominated atom.
Unlike the diffuse electron cloud that wraps around the nucleus, this central zone is compact, heavy, and acts as the gravitational anchor of atomic stability. The nucleus is composed of two fundamental types of subatomic particles: protons and neutrons. Protons carry a positive electric charge, with each proton equating to one unit of positive charge in the standard counting system, while neutral neutrons contribute no charge but add critical mass and stability.
The number of protons defines the atomic number, distinguishing one element from another. Neutrons, though electrically silent, play a vital role in buffering the repulsive force between protons through the strong nuclear force, a short-range but incredibly powerful interaction. “Without neutrons, atomic nuclei would be unstable,” explains physicist Helen Chen, “They act as nuclear glue stabilizing the atom’s structure.” The presence of the nucleus fundamentally shapes an atom’s identity and behavior.
Because nearly all an atom’s mass resides there—approximately 99.9%—the nucleus governs atomic weight and chemical reactivity. “We often act as if atoms are like billiard balls with electrons floating in space,” noted Dr. Rajiv Mehta, a nuclear physicist at MIT, “but it is the nucleus that truly dictates what an element can become.” The size of the nucleus varies by element—from hydrogen’s near-empty core with a single proton to uranium’s bloated nucleus containing 92 protons—but its influence remains undiminished.
Understanding the nature of the nucleus requires insight into quantum mechanics and nuclear forces. The strong nuclear force, which binds protons and neutrons together despite electromagnetic repulsion among protons, operates within femtometer scales—far smaller than the length of a hydrogen atom. This force is so powerful that if the nucleus were the size of a football stadium, a proton and neutron within it would be less than a few centimeters apart.
Quantum effects dictate stability patterns, leading to the concept of magic numbers—specific counts of protons or neutrons that create exceptionally robust nuclei, much like the stable electron shells in chemistry. Experimental techniques such as particle accelerators and deep-core spectroscopy have revealed the intricate dynamics within the nucleus. Scattering experiments, probing atoms with high-energy particles, have mapped the distribution of charge and mass, confirming theoretical models like the liquid drop model and shell model.
These frameworks help predict nuclear behavior, stability, and decay patterns—critical knowledge in fields ranging from energy production to medical imaging and astrophysics. Despite its minuscule size, the nucleus is a powerhouse. Its energy scales reflect billions of electron volt energies, fueling phenomena from stellar nucleosynthesis in stars to radioactive decay in equipment and medicine.
Radioactive isotopes, born from unstable nuclei, power medical diagnostics, cancer treatment, and even spacecraft power sources—a dramatic demonstration of these subatomic hearts in action. In sum, the center of an atom—the nucleus—is not merely a dense core but the dynamic epicenter of mass, charge, and nuclear forces. It defines the element’s very identity, governs its interactions, and underpins the stability that allows matter to exist as we know it.
Far from empty space, the nucleus is a quantum fortress where the rules of nature converge—shaping everything from the elements on the periodic table to the stars illuminating the cosmos.
The nucleus, though invisible to direct observation, is scientifically undeniable. Its structure continues to inspire discovery, revealing how the smallest realms hold the keys to the universe’s grandest phenomena.



Related Post
What Is the Center of an Atom Called? Unraveling the Core Mysteries of Atomic Structure
What They Don’t Want You to Know About Vitamin C: The Untold Science Behind This Controversial Nutrient

