What Is Alpha Decay: The Silent Transformation Inside Every Radioactive Atom
What Is Alpha Decay: The Silent Transformation Inside Every Radioactive Atom
Alpha decay is a fundamental process in nuclear physics, representing one of nature’s most precise and predictable mechanisms of radioactive disintegration. At its core, alpha decay occurs when an unstable atomic nucleus emits an alpha particle—essentially a helium-4 nucleus—allowing the parent atom to stabilize through atomic recalibration. This phenomenon not only unveils the inner workings of atomic nuclei but also plays a crucial role in energy production, medical diagnostics, and even environmental monitoring.
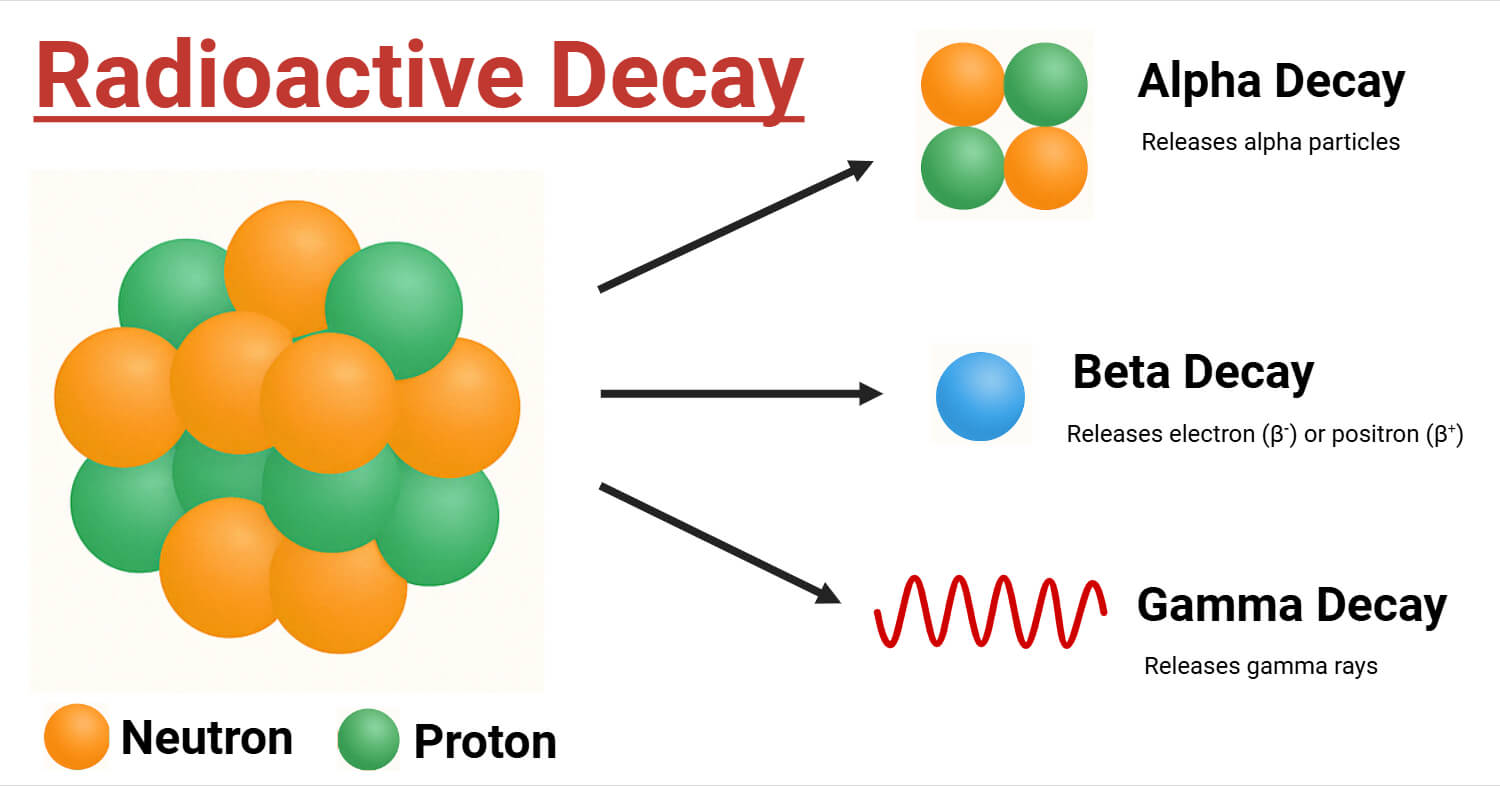
Defined scientifically, alpha decay is a type of radioactive decay in which an unstable nucleus releases an alpha particle composed of two protons and two neutrons—proven to be comparatively heavy and positively charged. When this occurs, the original nucleus transforms into a new element with an atomic number reduced by two and a mass number decreased by four. For example, uranium-238 undergoes alpha decay to form thorium-234: ^{238}_{92}U → ^{234}_{90}Th + ^{4}_{2}He This equation captures the precise atomic transformation that defines alpha decay.
The physical process behind alpha decay arises from quantum tunneling and electrostatic forces. Inside the atomic nucleus, protons repel each other due to Coulomb forces, creating instability. While classical physics predicts such repulsion should prevent breakdown, quantum mechanics reveals a pathway: alpha particles escape despite energy barriers by “tunneling” through them—a counterintuitive phenomenon first mathematically described by George Gamow in the 1920s.
“Alpha decay illustrates quantum mechanics in everyday reality—something invisible but measurable, shaping elements and driving decay chains,” notes Dr. Elena Vasiliev, a nuclear physicist at the Institute of Radiological Sciences. Measurement of alpha decay relies on detecting the emitted alpha particle or the resulting daughter nucleus.
Its range in matter—typically a few centimeters in air and just micrometers in solid materials—makes it highly penetrating in vacuum but easily blocked by biological tissue or a sheet of paper, which ironically enhances its practical radiological significance. Because alpha rays deposit enormous energy over short distances, they can cause severe cellular damage if ingested or inhaled, a factor central to radiation safety protocols. One of the most compelling aspects of alpha decay is its role in natural and artificial transmutation.
In geology, alpha decay powers radiometric dating methods, particularly in uranium-lead systems, enabling scientists to date rocks older than a billion years with remarkable accuracy. The 238U-234Th decay chain, for instance, serves as a cornerstone in chronometric studies of Earth’s crust. Beyond Earth, alpha emitters like radium-226—once used in luminescent paints—exemplify human utilization of this decay process, though such applications now demand strict regulation due to radiological hazard.
In medicine, alpha decay is increasingly exploited for targeted cancer therapies. Radionuclides such as actinium-225 and radium-223 decay via alpha particles, delivering highly localized radiation that selectively destroys malignant cells while sparing healthy tissue. This precision leverages the short range and high ionization density of alpha emitters, leading to improved treatment outcomes in advanced carcinomas.
As the National Institutes of Health (NIH) highlights, “Alpha therapies represent a frontier in radiopharmacology—where physics meets targeted therapy with transformative potential.” Understanding alpha decay also deepens insight into nuclear structure and the forces that bind nucleons. The decay rate, quantified by half-life, varies widely among isotopes—from nanoseconds to billions of years—reflecting the balance between binding energy and Coulomb repulsion in the nucleus. “Each alpha decay event is a tiny engine of transformation—proof that even the smallest quantum fluctuations influence the macroscopic world,” writes Dr.
Vasiliev. Modern research continues to refine detection techniques and harness alpha decay across interdisciplinary fields. Advances in germanium detectors and silicon tracking systems now allow precise measurement of alpha energies and particle trajectories, enhancing both fundamental research and applied nuclear safety.
Environmental monitoring relies on alpha spectrometry to detect contamination from nuclear fallout or industrial sources, offering early warnings of radiological threats. Beyond its technical applications, alpha decay challenges perceptions of stability—revealing that even the most seemingly immutable atoms are in constant flux, governed by invisible forces operating at subatomic scales. Its predictable yet elegant behavior underscores physics’ capacity to decode nature’s secrets at the most fundamental level.
From industrial diagnostics to planetary timelines and cancer treatment, alpha decay remains not just a scientific curiosity, but a cornerstone of modern nuclear science. What Is Alpha Decay: A Window Into Atomic Instability and Nuclear Transformation Alpha decay is far more than a laboratory curiosity—it is a fundamental process that reshapes matter at its most basic level. By emitting an alpha particle, unstable nuclei recalibrate energy and structure, offering scientists a direct window into quantum-scale dynamics.
As Dr. Vasiliev emphasizes, “Alpha decay puts us in contact with nature’s most precise clocks and scalpel—where radioactivity reveals life’s origins, guides medicine, and safeguards our future.” The transformation signifies both destruction and renewal: one nucleus fades, yet from its decay emerges a new element, a measurable timeline, and a tool of profound scientific and medical value. In every alpha particle released, lies a story of instability, transformation, and the silent power that governs the atomic world.



Related Post

Urgent Care Kit Com USA: Your Fast, Reliable Health Solution in Minutes

Samuel Joseph Mozes: A Biography That Defines Leadership, Innovation, and Resilience

