What Happens in G1: The Critical Cell Cycle Gatefire
What Happens in G1: The Critical Cell Cycle Gatefire
The G1 phase of the cell cycle stands as a pivotal checkpoint in cellular division, serving as much more than a simple pause between DNA replication and replication completion. It functions as a biological command center where cells assess internal and external signals, evaluate growth conditions, and determine whether to proceed with division or remain quiescent. This stretching summit of control influences cancer development, tissue repair, and developmental biology, making understanding what transpires during G1 indispensable.
What unfolds in this narrow window dictates whether a cell thrives, pauses, or halts—ultimately shaping the health and integrity of tissues across the body. During G1, the cell undergoes a series of tightly regulated molecular events that govern its readiness to commit to S phase—the stage where DNA synthesis begins. This delicate orchestration ensures accuracy: cells expand in size, double key metabolic and structural components, and secure sufficient resources before assuming irreversible responsibilities for replication.
According to molecular biologist Dr. Elena Maslov, “G1 is the gateway where a cell’s environment is analyzed like a quality control report before the engine of division starts.”
The Cellular Reset: Growth and Preparation
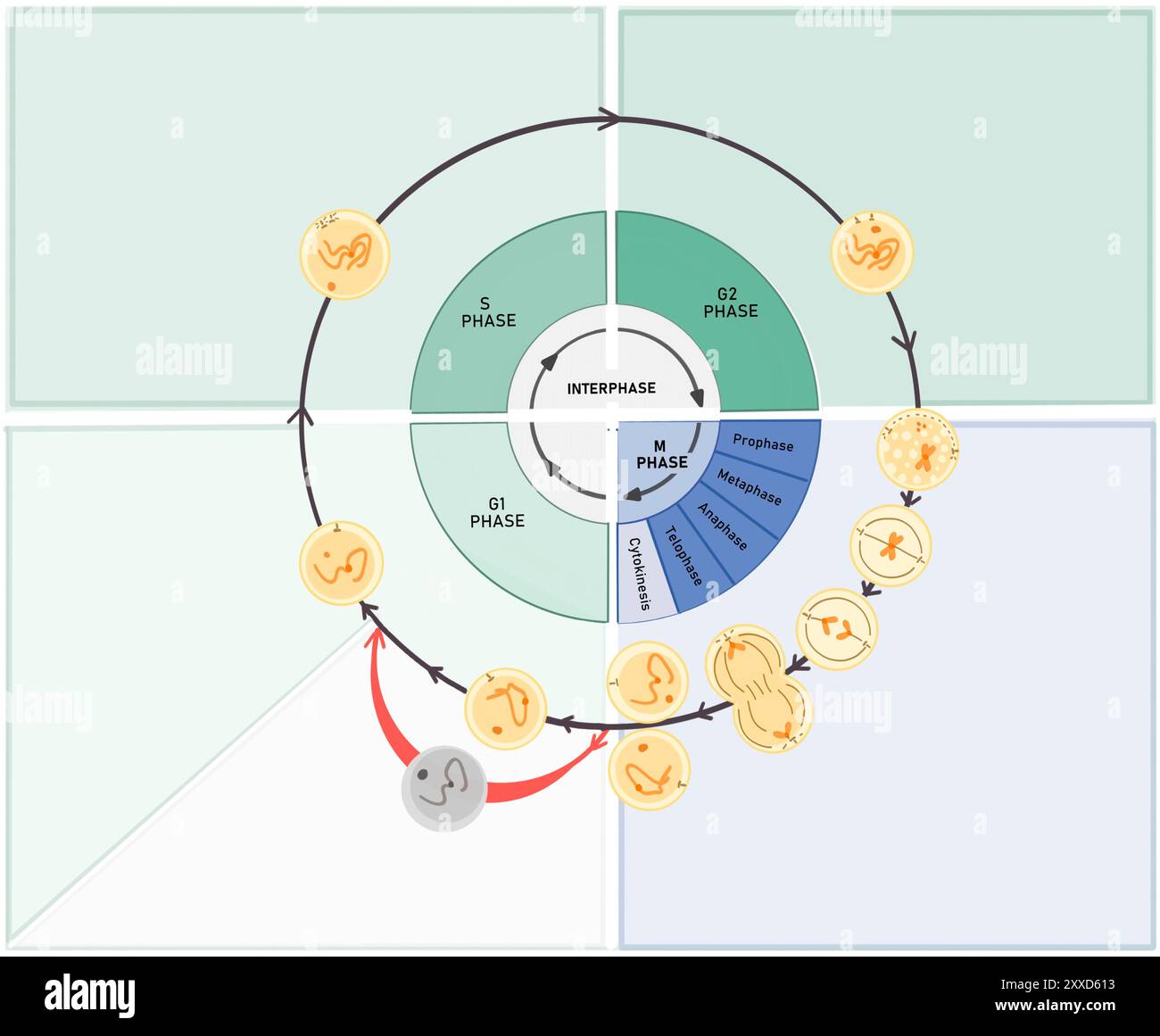
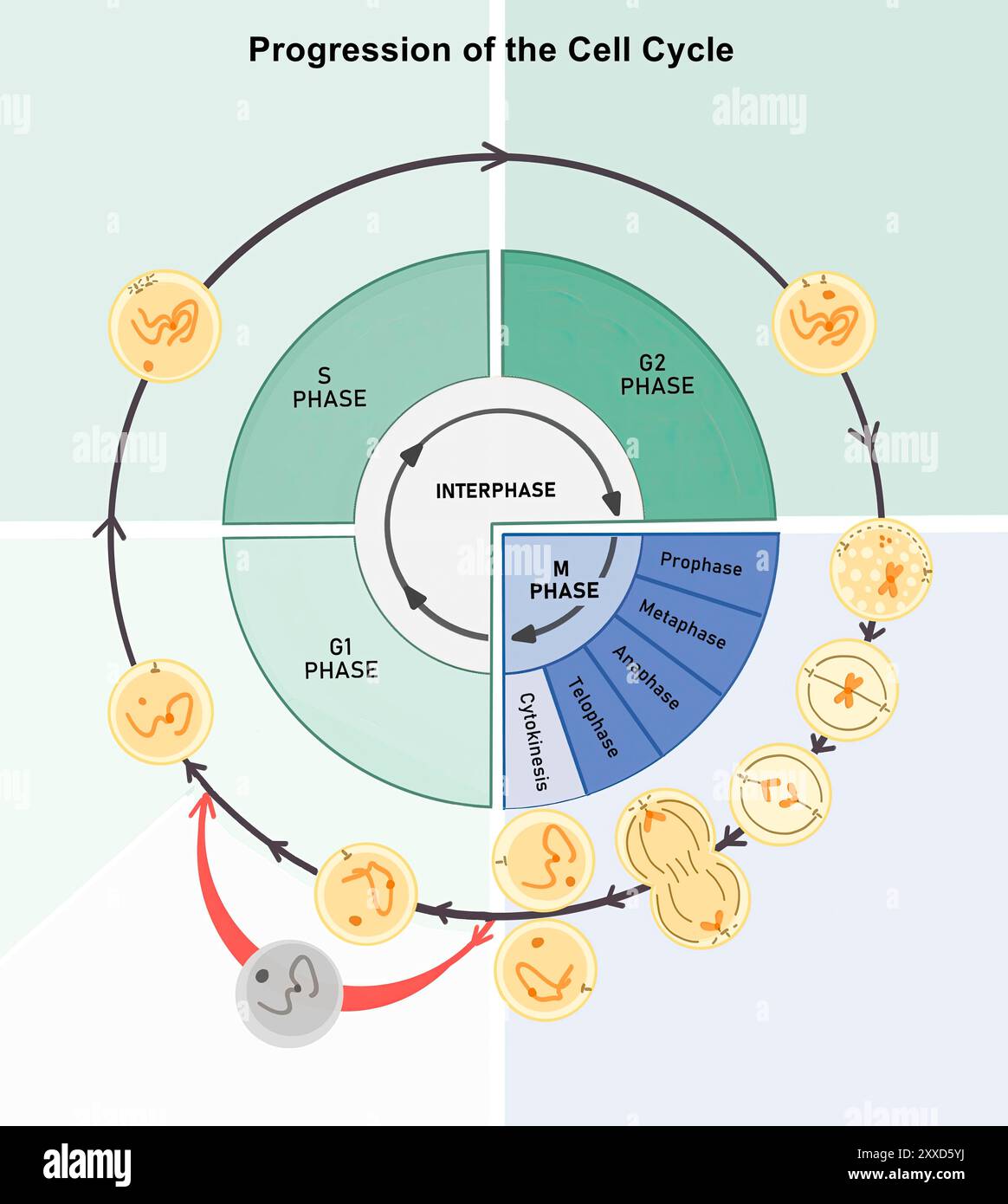
At the core of G1 lies cellular expansion—a phase defined by biosynthesis and growth. While S phase replicates the genetic blueprint, G1 allows the cell to roughly double in size, ensuring there is enough cytoplasm and organelles to support division.This growth phase is not passive; it involves active protein synthesis regulated by growth factors and nutrient availability. For example, insulin-like growth factors stimulate the PI3K-AKT signaling pathway, promoting cytoskeletal expansion and membrane enlargement. Crucially, G1 provides time for the cell to: - Synthesize essential enzymes and structural proteins needed later in the cycle.
- Replenish energy stores, primarily through glycolysis and mitochondrial activity. - Monitor nutrient levels, growth signals, and stress markers such as DNA damage. Without this preparation, cells risk division with insufficient resources, increasing mutation risk or triggering apoptosis.
As noted in recent reviews, “G1 sets the stage not just for replication, but for survival.”
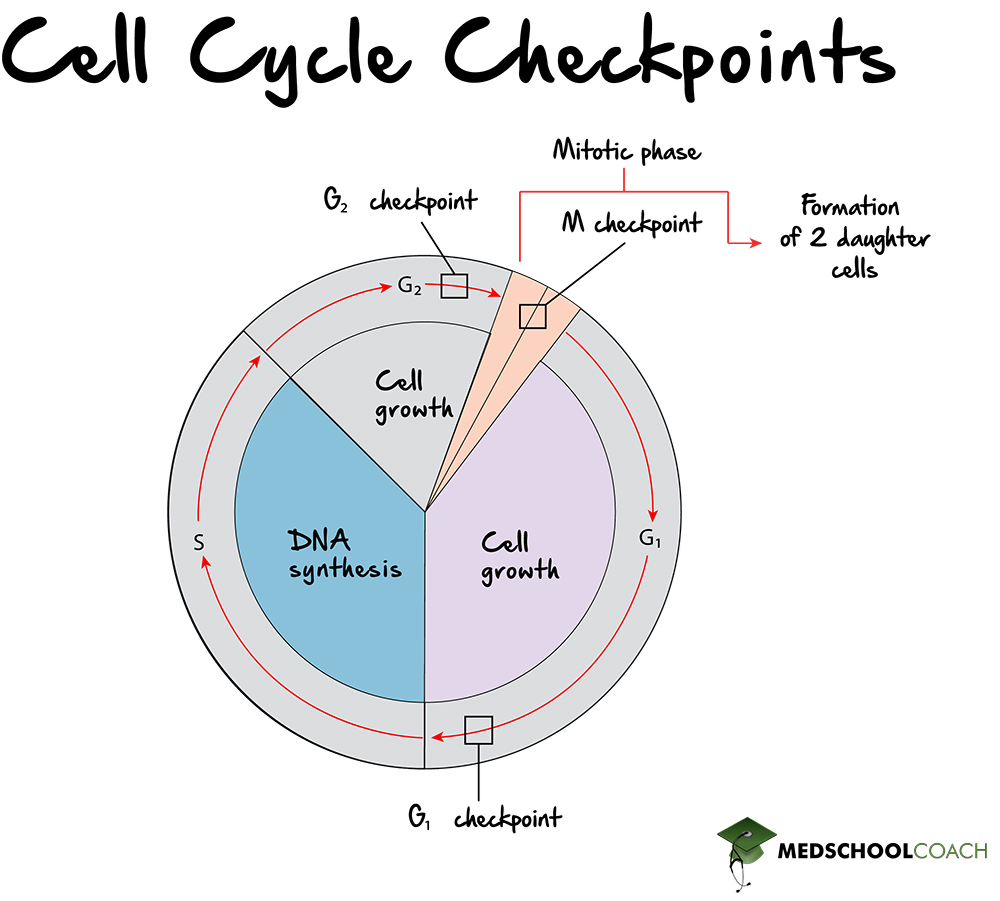
Checkpoints: Quality Assessment and Decision-Making
Immortalized within G1 is the function of biological surveillance—a network of checkpoints that scrutinize cell health before advancing to S phase. The G1 checkpoint, in particular, acts as a fail-safe that inspects DNA integrity, growth signals, and metabolic status. Key players include cyclin-dependent kinases (CDKs) and their regulatory partners—cyclins—whose precise timing controls entry into S phase.CDK4 and CDK6, bound to D-type cyclins, initiate the first rightward shift in the cell cycle by phosphorylating the retinoblastoma protein (Rb). When Rb is inactivated through phosphorylation, gene promoters such as E2F are unleashed, activating genes required for DNA replication. This transition is not automatic: - DNA damage triggers pathways like p53 activation, halting progression until repairs are complete.
- Insufficient growth factors or nutrient shortages dampen cyclin expression, delaying entry. “G1 is the brain of the cell cycle,” explains cell biologist Dr. Rajiv Nair, “where only cells fully prepared receive the go-ahead—preventing errors that could lead to cancer or tissue failure.”
Molecular Dynamics: Cyclins, CDKs, and Regulatory Complexes
The molecular engine driving G1 relies on cyclin-CDK complexes whose assembly and activity are rigorously controlled.- Cyclin D peaks early in G1 and pairs with CDK4/6, phosphorylating Rb to release E2F. - Cyclin E rises mid-to-late G1, reinforcing the transition and activating additional genes for replication. - Cyclin A binds later, supporting S phase entry as G1 closes.
These complexes do not act alone. Co-activators such as p300 and chromatin remodelers modify histones, preparing DNA for replication. Regulatory proteins including p21 and p27 serve as brakes, responding to stress signals and ensuring no premature progression.
G1 is also a phase of metabolic readiness: - Cells shift from oxidative phosphorylation to aerobic glycolysis, providing rapid energy and intermediates for biosynthesis. - Mitochondrial function adapts to support increased biosynthetic output. Environmental inputs—oxygen levels, growth factors, and oxygen-sensing pathways like HIF-1—tune these processes to align division with physiological needs.
Gene Expression and Transcriptional Control
Transcriptional regulation is central to G1 function. Key transcription factors drive expression programs essential for cell cycle progression. - E2F transcription factors, released from Rb inhibition, activate genes involved in DNA replication, cell cycle progression, and anti-apoptotic proteins.- MYC amplifies expression of growth-promoting genes, reinforcing cellular expansion. Transcriptomic studies reveal G1 as a dynamic transcriptional landscape, where genes are activated or repressed in a precisely timed sequence. Epigenetic modifications, including DNA methylation and histone acetylation, further refine this gene expression, ensuring the cell’s identity and readiness are preserved.
Challenges such as hypoxia, DNA damage, or oncogenic signals disrupt this balance. Cells with activated oncogenes or inactivated tumor suppressors may bypass G1 checkpoints, fueling uncontrolled division—highlighting the phase’s role as both guardian and battleground in cancer biology.
Clinical and Biological Significance
The importance of G1 extends far beyond basic cell biology into medicine and disease.In cancer therapy, targeting G1 regulators offers promising strategies. CDK4/6 inhibitors, such as palbociclib, block progression by preventing Rb phosphorylation, effectively trapping cells in G1. These drugs have transformed treatment for certain breast cancers, underscoring G1’s vulnerability as a therapeutic target.
Beyond oncology, G1 influences tissue homeostasis and regenerative capacity. Stem cells exploit G1 dynamics to balance self-renewal and differentiation—critical for tissue repair and development. Disruptions during development—whether through premature G1 exit or prolonged entry—can lead to growth defects or disease.
Ongoing research probes how metabolic shifts, epigenetic marks, and stress responses shape G1 outcomes. Understanding these layers deepens insights into aging, regenerative medicine, and the prevention of age-related decline.
What unfolds in G1 transcends mere cell division; it is a master regulator of cellular fate, a quantum checkpoint where life’s most fundamental decisions are made.
From orchestrating growth and surveillance to balancing repair with repair, this phase shapes the integrity of every organism. As science advances, G1 remains a frontier of discovery—not just in biology, but in medicine’s most pressing quests.

Related Post
Stalker SoC: Unveiling The Secrets Of X19

Unlocking The Sweetness: What Is Honeytoon?

