What Experiment Did Niels Bohr Conduct—and How It Revolutionized Atomic Physics
What Experiment Did Niels Bohr Conduct—and How It Revolutionized Atomic Physics
In a pivotal moment of 20th-century science, Niels Bohr conducted a groundbreaking experiment that redefined the understanding of atomic structure. His work laid the foundation for modern quantum mechanics by revealing how electrons orbit the nucleus under strict, quantized rules. More than a technical insight, Bohr’s experimental approach fused theoretical boldness with empirical precision, reshaping how physicists perceive the infinitesimal world.
The experiment, rooted in early quantum theory, demonstrated that atomic stability arises not from classical physics alone, but from rare, fundamental quantum conditions—proving that electrons behave unlike particles in everyday experience.
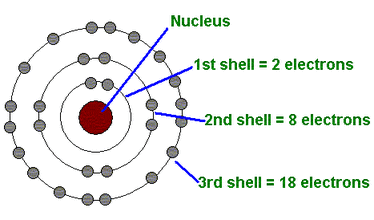
At the heart of Bohr’s investigation was the stability of the hydrogen atom, which classical electromagnetism predicted should collapse as orbiting electrons radiate energy and spiral into the nucleus. To resolve this paradox, Bohr proposed a radical model—now known as the Bohr model—integrating Rutherford’s nuclear atom with Planck’s quantum hypothesis.
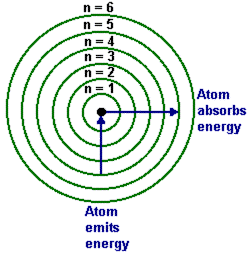
He postulated that electrons occupy discrete, stable orbits where angular momentum is quantized, satisfying the rule: angular momentum = nℏ (n = 1, 2, 3, ...). “Only those orbits in which the electron’s angular momentum is an integer multiple of ℏ are permitted,” Bohr wrote in his 1913 paper, a statement that became a cornerstone of quantum physics. "The stability of matter hinges on these discrete transitions—electrons do not radiate continuously, but jump in quantized steps."
To test his hypothesis, Bohr combined classical mechanics with early quantum ideas.
His experimental framework centered on measuring the hydrogen spectrum—specifically the visible line spectrum consisting of sharp wavelengths emitted when electrons transition between energy levels. Using spectroscopic data from screenings of hydrogen discharge tubes, he linked observed spectral lines directly to electron energy changes: each transition corresponds to emission or absorption of a photon with precisely defined energy \(E = h\nu\), where \(h\) is Planck’s constant. For the famous Balmer series—responsible for the red and blue lines in hydrogen’s spectrum—Bohr derived a formula predicting exact wavelengths based on quantum jumps.
The match between his calculation and experimental records was exact, offering compelling evidence for his model.
- Electrons occupy fixed, quantized orbits with angular momentum \(L = n\hbar\)
- Electrons do not radiate energy while in stable orbits, preserving atomic integrity
- Light emission or absorption occurs only during discrete “jumps” between energy levels
- The frequency of emitted light corresponds to energy differences—verified by spectral line measurements
Bohr’s experiment transformed atomic theory by replacing deterministic orbits with probabilistic quantized states, marking a decisive break from Newtonian physics. His insight that atomic stability depends on quantum rules remains central to quantum chemistry, semiconductor physics, and atomic clocks.
Though later refined by Schrödinger’s wave mechanics and Heisenberg’s uncertainty principle, Bohr’s experimental approach established the critical link between theory and observable phenomena. His model, though simplified, demonstrated that nature follows exact mathematical laws at quantum scales—laws accessible only through bold conceptual leaps supported by empirical data. Bohr’s lasting legacy lies not in creating a perfect atomic blueprint, but in proving that the atom’s behavior obeys a hidden order.
The experiment inspired generations of physicists and revealed electrons not as classical orbiting bodies, but as quantized excitations governed by probability. Today, quantum technologies—from lasers to quantum computing—owe a substantial debt to Bohr’s experimental courage and theoretical foresight. What began as a focused test of atomic behavior evolved into a foundational pillar of modern physics, proving that even small systems harbor profound, universal principles.1
Bohr’s Experimental Synthesis: Theory Meets Observation
Bohr’s strength lay in reformulating theory through experimental validation.By taking hydrogen’s spectral emissions as a real-world constraint, he anchored abstract quantum assumptions in observable reality. His approach demonstrated that quantum mechanics must be tested, not merely inferred—a principle still guiding high-energy physics and quantum experimentation. The Bohr model, though superseded, remains a teachable milestone because it clearly illustrates how quantization prevents atomic collapse and explains spectral order.
The process underscored a broader shift: physics increasingly required not just mathematical elegance, but experimental rigor that forced theory to conform to measurement. Bohr’s work cultivated this ethos, making empirical evidence the ultimate arbiter of quantum reality. As modern experiments probe even smaller scales—via particle colliders and quantum sensors—the original insight endures: nature’s most fundamental workings unfold not in continuity, but in discrete, quantized leaps.2
The Enduring Impact on Science
Bohr’s experiment did more than solve a technical puzzle—it redefined the scientific method in atomic physics.By tying theoretical postulates to precise spectroscopic data, he established a model of inquiry where quantum hypotheses are validated through observable consequences. This principle underpins all modern quantum research, from quantum optics to topological materials. It also opened doors to predicting unknown phenomena—Bohr’s quantized orbits foreshadowed the discovery of isotopes, electron spin, and quantum tunneling.
In retrospect, Niels Bohr’s experiment stands as a landmark not just for its results, but for its methodology. It showed that breakthrough science emerges when bold theory is relentlessly tested against nature’s quiet_data. Today, as quantum computing and precision measurement advance, Bohr’s fusion of experiment and insight continues to inspire.
His work reminds scientists and the public alike that even in the smallest realms of matter, profound laws await discovery through courage, clarity, and careful observation.
- Bohr merged classical orbits with quantum rules—ushering in a new physics era. - His approach set the standard


Related Post

Behind the Innovation: Ado Den Haag Transforming Digital Experiences with Uncompromising Vision
Iflix Download Apk For Android: Unlock Seamless Streaming Quality on the Go

Are Sandra Oh and Ellen Pompeo Still Friends? The Public Glimmer of Affection in a Subtle Bond

Bates Motel: Where Horror Lurks Behind The Motel Sign, One Haunting Frame at a Time

