Unveiling the Atomic Bond: The Lewis Dot Structure of Hydrogen Cyanide and Its Electron Dance
Unveiling the Atomic Bond: The Lewis Dot Structure of Hydrogen Cyanide and Its Electron Dance
Hydrogen cyanide (HCN), a compound of stark simplicity and profound chemical significance, reveals its structural elegance through its Lewis dot structure. This fundamental representation of shared electrons not only illuminates HCN’s molecular geometry but also underpins its reactive properties and ethical importance in synthetic chemistry and industrial applications. More than just a sketch of atoms and lines, the Lewis dot structure of HCN offers a window into the invisible forces shaping molecular behavior—where electron distribution dictates function.
This detailed exploration delves into the precise bonding patterns, formal charges, and chemical implications derived from its simplified atomic visualization.
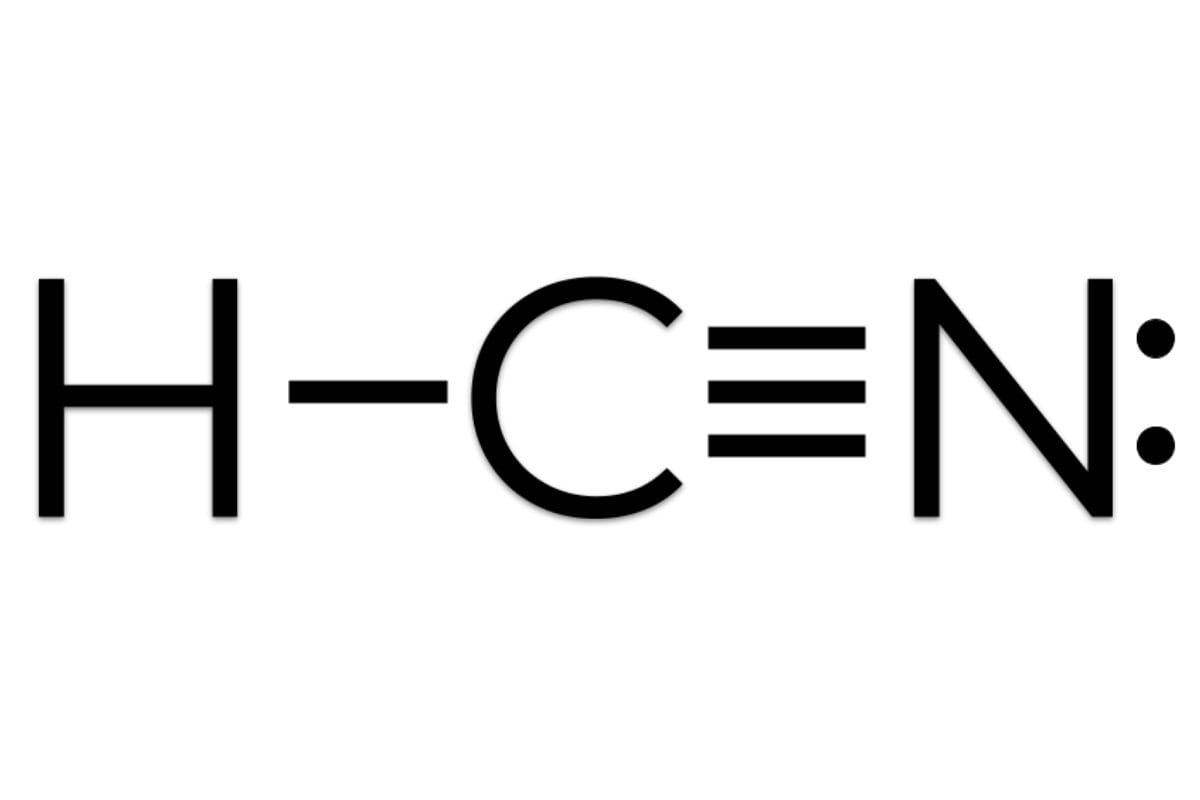
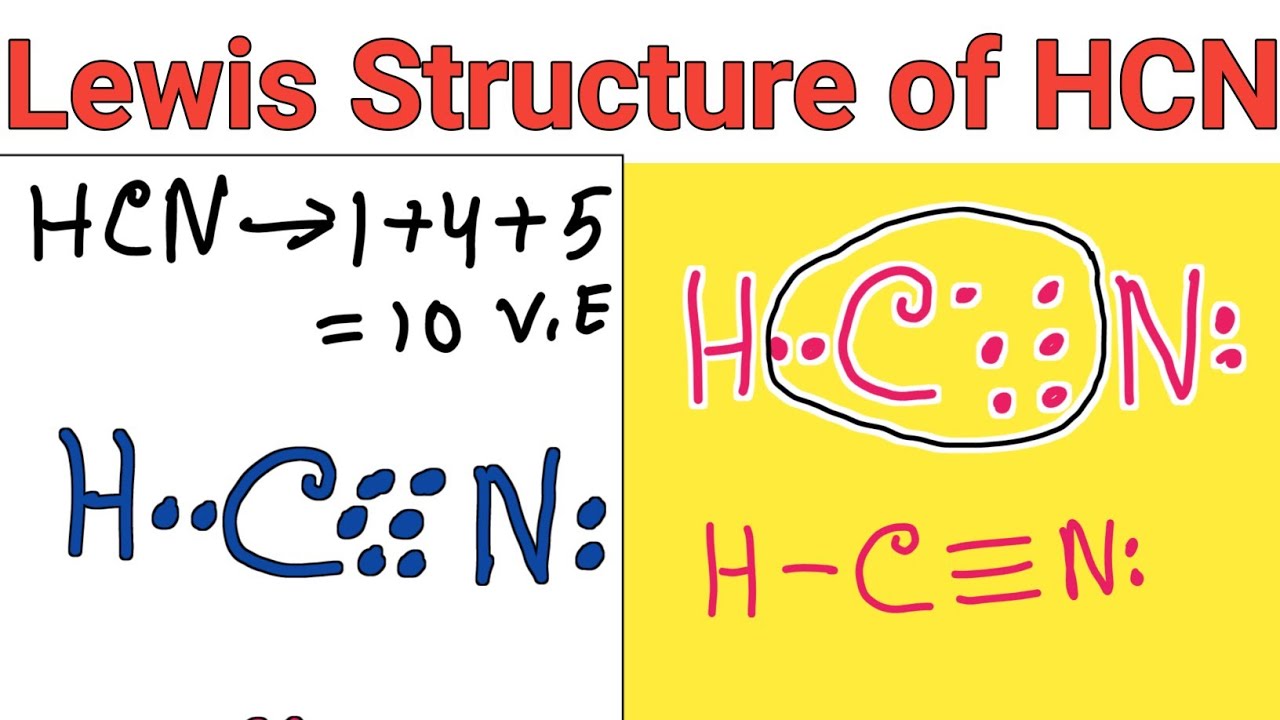
The Lewis dot structure of hydrogen cyanide begins with its core components: one hydrogen atom covalently bonded to a nitrogen atom, which is itself triple-bonded to a carbon atom, culminating in a terminal hydrogen connected to carbide. The hydrogen molecule contributes only one valence electron, while nitrogen and carbon each bring five and four respectively.
The central carbon forms a triple bond with nitrogen, a staggering five shared electrons (three shared via bond and two as lone pairs in resonance-stabilized configurations), while nitrogen delivers a strong partial negative character and weak nitrogen lone pair contributes to polar reactivity. Hydrogen’s single electron completes a shared pair with carbon, fulfilling octets indirectly through its molecular environment. The resulting structure—H−C≡N—embodies both stability and reactivity, defining HCN’s unique role in organic synthesis and industrial chemistry.
Atomic Arrangement and Bonding Dynamics in Hydrogen Cyanide
The molecular architecture of hydrogen cyanide is defined by a strikingly precise arrangement: HCN adopts a linear geometry with a central carbon atom at the core. This structure arises from the triple bond—comprising one sigma (σ) bond and two pi (π) bonds—between carbon and nitrogen, a bond type renowned for its strength and directional precision. The triple bond exemplifies the synthesis of atomic orbitals, where carbon’s sp hybridization enables optimal orbital overlap along a single axis.In this hybridized state, carbon directs its four valence electrons efficiently into bonding, while nitrogen contributes five—three via bonding and two as lone pairs—facilitating both resonance and charge stabilization. Hydrogen, lacking lone pairs in this example, participates with a single electron to complete the shared pair with carbon. This minimal involvement underscores hydrogen’s role as a reactive binder rather than a lone participant.
The electron distribution follows the octet rule strictly at nitrogen and carbon, with nitrogen maintaining a formal negative charge in theoretical representation due to its higher electronegativity and lone pair resonance, while carbon retains near-neutral character. The triple bond’s electron density creates a dense region of negative charge around nitrogen, making it a vulnerable site for electrophilic attack—a key factor in HCN’s toxic mechanism and synthetic utility.
Key features of HCN’s Lewis dot structure include: - A triple bond (C≡N) binding carbon to nitrogen, delivering eight electrons in three shared pairs, stabilized by piano-stew keys hybridization.
- Nitrogen holding one lone pair and a formal negative charge in canonical form, enhancing electrophilic reactivity. - Hydrogen’s single electron shared with carbon, completing the bonding pair and anchoring the terminal atom. - Resonance contributions from nitrogen’s lone pairs improve structural stability and delocalization.
- A clear indication of polar character, with nitrogen electronegative enough to attract electrons, influencing molecular dipole and reactivity.
Formal Charges and Their Impact on Stability and Reactivity
In predicting molecular stability and reactivity, formal charges derived from Lewis dot structures serve as indispensable tools. Despite the triple bond’s complexity, formal charges across HCN reveal a remarkably neutral framework: carbon carries a formal charge of +2, nitrogen a formal charge of 0, and hydrogen a formal charge of 0.This distribution arises because carbon donates electrons to form both the sigma and pi bonds, accumulating a net positive charge, while nitrogen’s lone pair separation and electronegativity balance this charge across the molecule. Understanding Formal Charge Minimization is central to evaluating HCN’s behavior. Formal charges approximate electron assignment more accurately than simple Lewis structures, revealing that the actual electron distribution favors charge separation only where necessary.
The near-neutral nitrogen center, stabilized by lone pairs and resonance, reduces overall reactivity compared to more charged analogs. This subtle charge distribution enhances HCN’s role as a versatile nucleophile—critical in processes like cyanide poisoning mechanisms, esterification, and nucleophilic addition reactions.
Highlighting practical implications: - Formal charge neutrality at nitrogen contributes to HCN’s low volatility relative to similar small molecules, despite its high evaporation tendency.
- Electron-deficient environment around carbon enables HCN to act as both donor and acceptor in complex reactions, critical in pharmaceutical synthesis. - The structure predicts weak acidity at the hydrogen atom—evidenced by its partial ionization in solution—though the conjugate cyanide anion remains relatively stable due to resonance stabilization. - Charge visualization clarifies HCN’s ability to form salts (e.g., sodium cyanide) via ionic bonding with strong acids, a reaction foundational to industrial chemistry.
The Real-World Significance of HCN’s Structural Simplicity
The elegance of HCN’s Lewis dot structure translates directly into profound industrial and biological relevance. As a building block of organic synthesis, its triple bond enables transformations into amides, carboxylic acids, and heterocycles—compounds essential to pharmaceuticals, agrochemicals, and materials science. In polymer chemistry, HCN-derived precursors contribute to the manufacture of specialty resins and coatings.Yet, its molecular simplicity belies potential danger: HCN is a potent cyanide donor, able to inhibit cellular respiration by binding cytochrome c oxidase—a toxicity underscored by its Lewis structure’s direct pathway for electron transfer to biological heme centers. Ethical and Safety Considerations are thus inseparable from HCN’s utility. Industry safety protocols rely on understanding its molecular fragility—hydrogen’s labile bond with carbon allows unintended cleavage under stress, releasing toxic cyanide ions.
Proper handling demands rigorous containment and exposure controls, limiting occupational risks. Yet in carefully managed settings, HCN’s reactivity drives innovation, from the synthesis of life-saving drugs to sustainable chemical manufacturing.
Key takeaways include: - The Lewis dot structure reveals HCN’s triple bond as a cornerstone of stability and reactivity.
- Charge distribution supports HCN’s role as a selective yet aggressive nucleophile. - Structural insights explain its applications across synthesis, materials, and toxicology. - Balancing hazard and utility calls for precision in handling, informed by atomic-level clarity.
Hydrogen cyanide’s Lewis structure is far more than a static diagram—it is a dynamic guide to molecular behavior, connecting fundamental chemistry to real-world consequences. By decoding the language of electrons, scientists unlock safer, smarter usages of this compound, illuminating the hidden choreography of valence electrons binding atoms into matter with purpose.


Related Post
Here Is The Real Meaning Behind Erome Mp4 3

Unlock Sutty Hollywood’s Database: Master the HCarchanswers Homepage Login via YouTube Step-by-Step

Ian Garry Wife: Behind the Public Figure – The Untold Story of a Private Life Shaped by Partnership and Resilience
Natasia Demetriou Husband: Relationship Status Explained — The Truth Behind the What We Do in the Shadows Star’s Private Life

