Unraveling Energy Power: The Essential Mechanism of Oxidative Phosphorylation
Unraveling Energy Power: The Essential Mechanism of Oxidative Phosphorylation
At the very core of cellular energy production lies oxidative phosphorylation—a biochemical process that converts the chemical energy from electrons into usable ATP, the cell’s primary energy currency. As explained in key Pogil answer restored frameworks, this intricate pathway orchestrates the transfer of energy derived from nutrients through a series of protein complexes embedded in the inner mitochondrial membrane. Without oxidative phosphorylation, life’s fundamental processes—from muscle contraction to nerve signaling—would collapse.
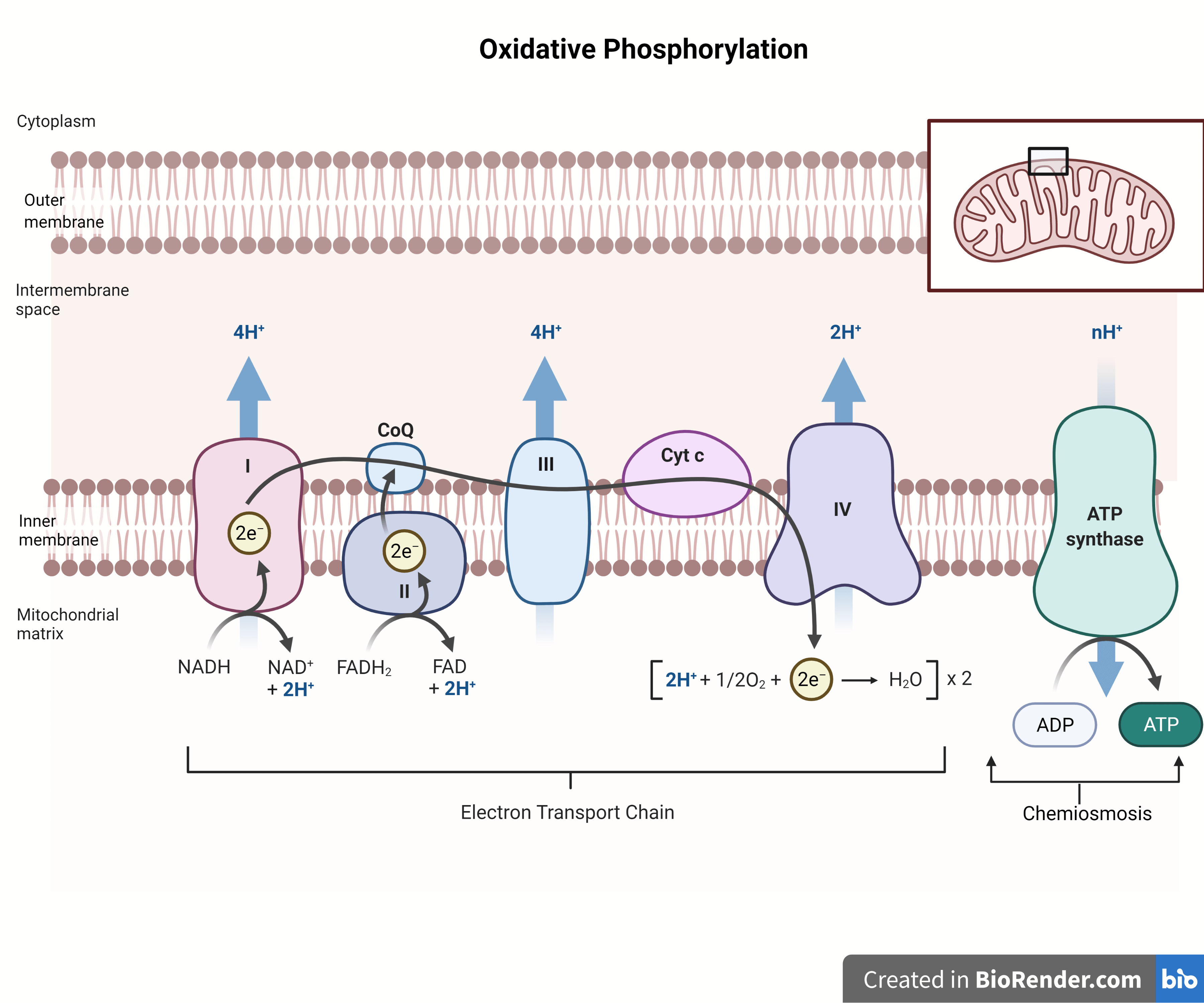
This article examines the central concepts of oxidative phosphorylation using insights from Pogil-style explanations, revealing how protons, electrons, and molecular machinery converge to fuel every living cell. The process begins with electron transport, a chain of redox reactions spanning four multi-subunit protein complexes—commonly referred to as Complexes I through IV.
Electron Transport and Proton Pumping
Electrons, donated primarily by NADH and FADH₂ from earlier stages of cellular respiration, enter the electron transport chain at Complex I and Complex II.As these high-energy electrons pass through the complexes, their energy is harnessed to actively pump hydrogen ions (H⁺) from the mitochondrial matrix into the intermembrane space. This creates a steep electrochemical proton gradient—often called the proton-motive force—where both a concentration difference and an electrical potential build up across the membrane. “Every electron transfer step is coupled to proton translocation; energy flows from redox potential to mechanical work,” explains the Pogil framework, emphasizing precise molecular coordination.
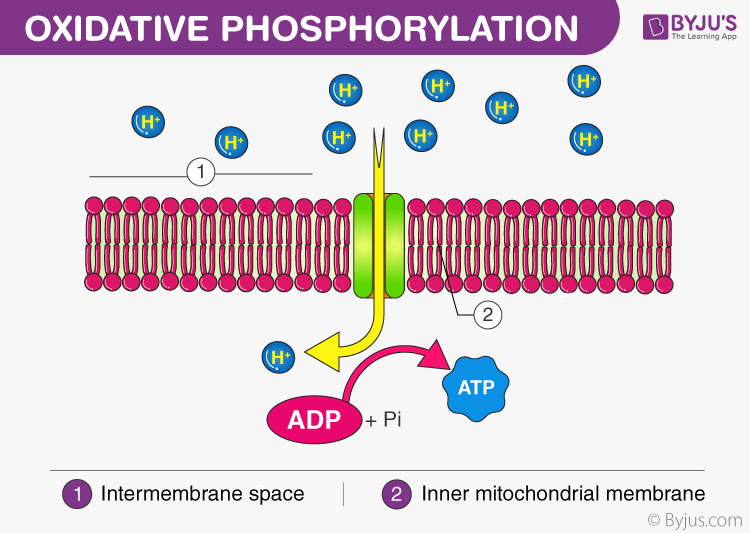
As protons pass through the Fo complex, they induce conformational changes in the Fo stalk, which rotate the central γ-subunit within F1. This rotation triggers precise structural shifts that bind ADP and inorganic phosphate (Pi), facilitate their condensation into ATP, and release the newly formed molecule into the matrix. This elegant mechanism, captured in Pogil diagrams and conceptual explanations, demonstrates nature’s mastery of energy conversion at the molecular level.
“A remarkable feat of biological engineering—energy harnessed from ion flow powers a molecular machine that synthesizes thousands of ATP molecules per second,” notes a core principle emphasized in advanced cellular biology resources.
The Integration of Redox Chemistry and Mechanics
Oxidative phosphorylation exemplifies seamless integration between redox biochemistry and mechanical force generation. The electron carriers NADH and FADH₂ shuttle high-energy electrons derived from glycolysis, the Krebs cycle, and fatty acid oxidation to Complex I and Complex II, respectively.As electrons move down their redox potential, energy released is precisely channeled to pump protons—no “leakage,” no wasted kinetic energy. This elegant coupling ensures maximal efficiency. The Stoichiometry of proton pumping per electron pair is tightly regulated; typically, one NADH yields approximately 2.5 ATP, while FADH₂ generates about 1.5 ATP, reflecting differences in entry points and coupling efficiency.
The Pogil cell models vividly depict ATP synthase as a needle in a gear system: the flow of protons powers a mechanical rotation, which then drives catalytic conformations with astonishing specificity and speed.
This tightly regulated mechanism underscores why oxidative phosphorylation remains the primary ATP generator in aerobic organisms, accounting for up to 90% of cellular energy output in metabolically active tissues like heart and brain. Understanding each step—from electron flow to mechanical catalysis—provides not only insight into cellular physiology but also critical perspectives for research in bioenergetics, metabolic disease, and biotechnological energy applications.
In summary, oxidative phosphorylation is the final, indispensable juncture in cellular respiration where electrons yield ATP through a masterpiece of bioenergetic design.
Its principles, masterfully distilled in Pogil learning tools, illuminate how life converts invisible chemical energy into the tangible power that sustains living systems. Mastery of these concepts enables a deeper appreciation of biology’s elegant machinery—where redox reactions fuel the world’s most essential molecule, ATP.


Related Post
Exploring The Life Of Brigitte Macron: Young Years, Influence, and Lasting Legacy

Unlock Somalia’s Digital Frontier: How Telegram is Revolutionizing Communication and Business
Decoding the Chinese 1973 Zodiac: A Deep Dive into the Water Tiger Year

