Unlocking the Structure of Air: The Molecular Weight of N₂ and Its Scientific Significance
Unlocking the Structure of Air: The Molecular Weight of N₂ and Its Scientific Significance
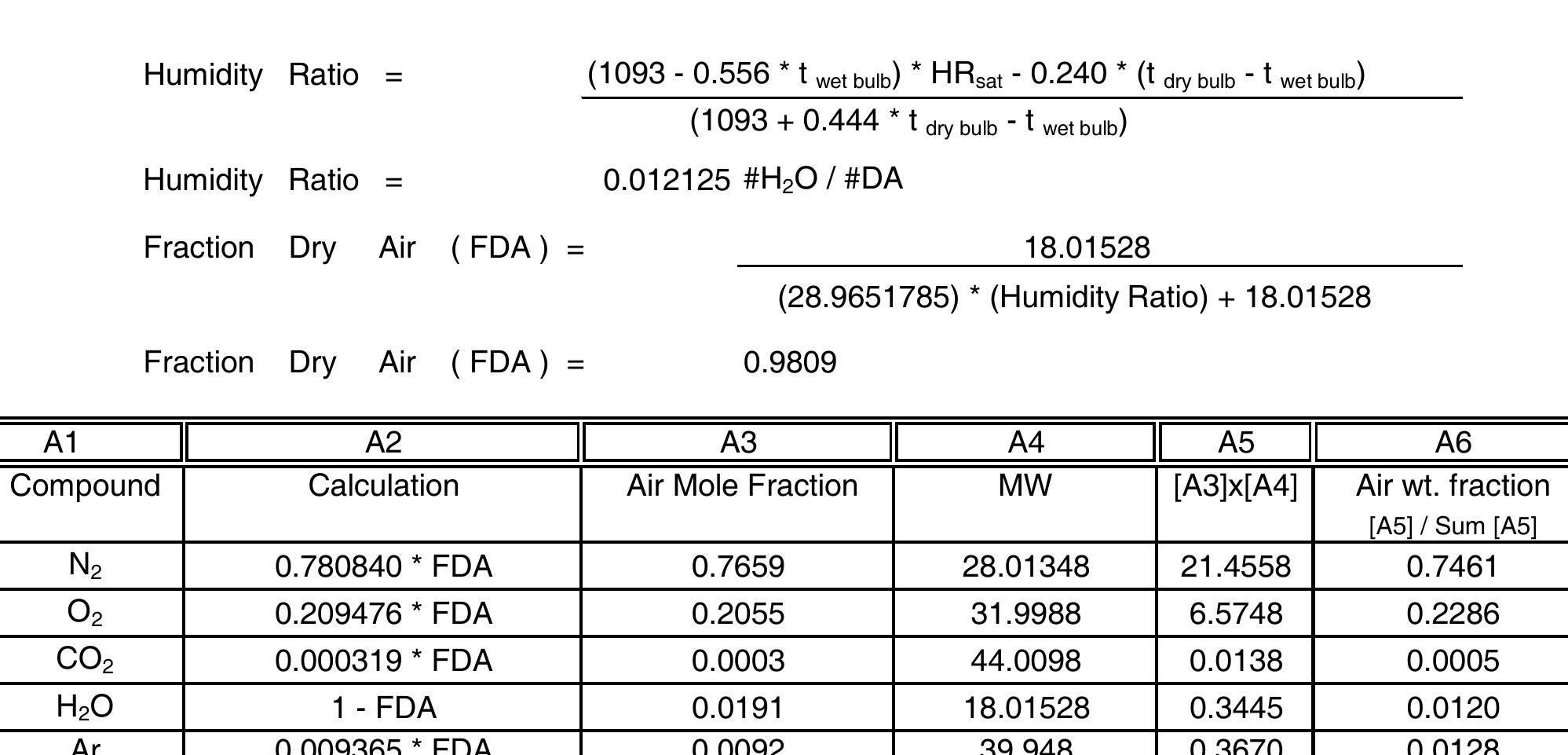
Molecular weight of N₂, a deceptively simple diatomic molecule, forms the backbone of atmospheric composition and underpins critical chemical and biological processes. With a precise molecular weight of 28.014 grams per mole, nitrogen gas (N₂) remains central to chemistry, environmental science, and industrial applications—from fertilizer production to rocket fuel stabilization. Understanding this value unlocks deeper insight into why nitrogen dominates Earth’s atmosphere at approximately 78%, and how its inertness shapes both natural and engineered systems.
Nitrogen exists almost entirely as N₂ in the troposphere, a result of its strong but chemically selective triple bond. The molecular weight of N₂—calculated as (14 g/mol × 2) since each nitrogen atom contributes 14 atomic mass units—defines not just its mass but also its behavior in chemical reactions. “N₂ is remarkable for its stability,” explains Dr.
Elena Petrova, a physical chemist at MIT. “That high molecular weight contributes to its low reactivity compared to single atoms, because energy is required to break the triple covalent bond under ambient conditions.”
This molecular robustness directly influences atmospheric dynamics. At standard temperature and pressure (STP), N₂ molecules average a mass that keeps them relatively dense yet mobile in the gas phase.
Despite its weight, N₂ circulates globally via wind patterns, maintaining equilibrium in a system where slight fluctuations in concentration affect climate and weather systems. The 28.014 atomic mass unit value thus anchors climate models predicting nitrogen’s role in greenhouse gas interactions and stratospheric ozone chemistry.
Beyond Earth, nitrogen’s molecular weight influences planetary science.
In the cold expanses of interplanetary space, N₂ remains a stable component even at temperatures near absolute zero. Its low volatility—driven by molecular mass—keeps it bound in icy bodies and atmospheres, playing a key role in models of solar system formation and exoplanet atmospheric chemistry. “Molecular weight tells us how nitrogen survives extreme environments,” notes planetary scientist Dr.
Rajiv Mehta. “Its triple bond and 28-unit mass make it a robust tracer for atmospheric escape and planetary evolution.”
In industrial contexts, precise knowledge of N₂’s molecular weight is essential. Compressed nitrogen, widely used in medical, manufacturing, and food preservation, is stored and transported with exacting pressure norms based on this value.
The 28.014 atomic weight ensures accurate calculations for gas mixtures, pipeline flows, and thickening processes where nitrogen acts as an inert diluent. In semiconductor fabrication, where oxygen exclusion is critical, nitrogen’s molecular properties—defined by its mass and bond strength—enable cleanroom environments free of reactive contaminants.
Quantifying N₂’s molecular weight also enhances scientific research.
In mass spectrometry, nitrogen compounds are identified by their precise ratios, and understanding N₂’s exact 28.014 atomic mass unit enables accurate calibration of instruments used in everything from drug development to environmental monitoring. In laboratory chemistry, reactions involving nitrogen gas rely on consistent mass data to predict yields and thermodynamic behavior, ensuring reproducibility across experiments.
In summary, the molecular weight of N₂—28.014 atomic mass units—is far more than a number.
It encapsulates nitrogen’s chemical identity, atmospheric longevity, industrial utility, and cosmic endurance. From the breath of every living being to the engines of industry and the atmospheres beyond, this value grounds our understanding of one of Earth’s most vital elements. Reliable data on N₂’s molecular weight remains a foundational pillar in both theoretical science and applied technology,



Related Post

<strong>Lynn Martinez on Channel 7: Unveiling the Story Behind Her Age, Height, and Public Impact</strong>

Joey Essex’s Stage Dominance: How His Shows Redefined UK Variety Entertainment

What Does Cooking With Crisco Mean? The Surprising Science Behind America’s Shelf-Stable Staple

