Unlocking the Secrets of Gases: How Ideal Gas Constant R Shapes Modern Science
Unlocking the Secrets of Gases: How Ideal Gas Constant R Shapes Modern Science
At the core of thermodynamics and physical chemistry lies a deceptively simple constant: the Ideal Gas Constant, IdealGasConstantR. For engineers, chemists, and physicists, this numerical constant—approximately 8.314 joules per mole per kelvin—is not just a formula step, but a bridge between theoretical predictions and real-world gas behaviors. From weather forecasting to spacecraft propulsion, IdealGasConstantR quantifies how temperature, pressure, volume, and moles of gas interrelate, enabling precise engineering and groundbreaking research.
Using this constant, scientists decode complex atmospheric phenomena, optimize industrial processes, and simulate life-support systems—each application anchored in the elegant simplicity of ideal gas laws.
The Foundation: Understanding Ideal Gas Behavior and IdealGasConstantR
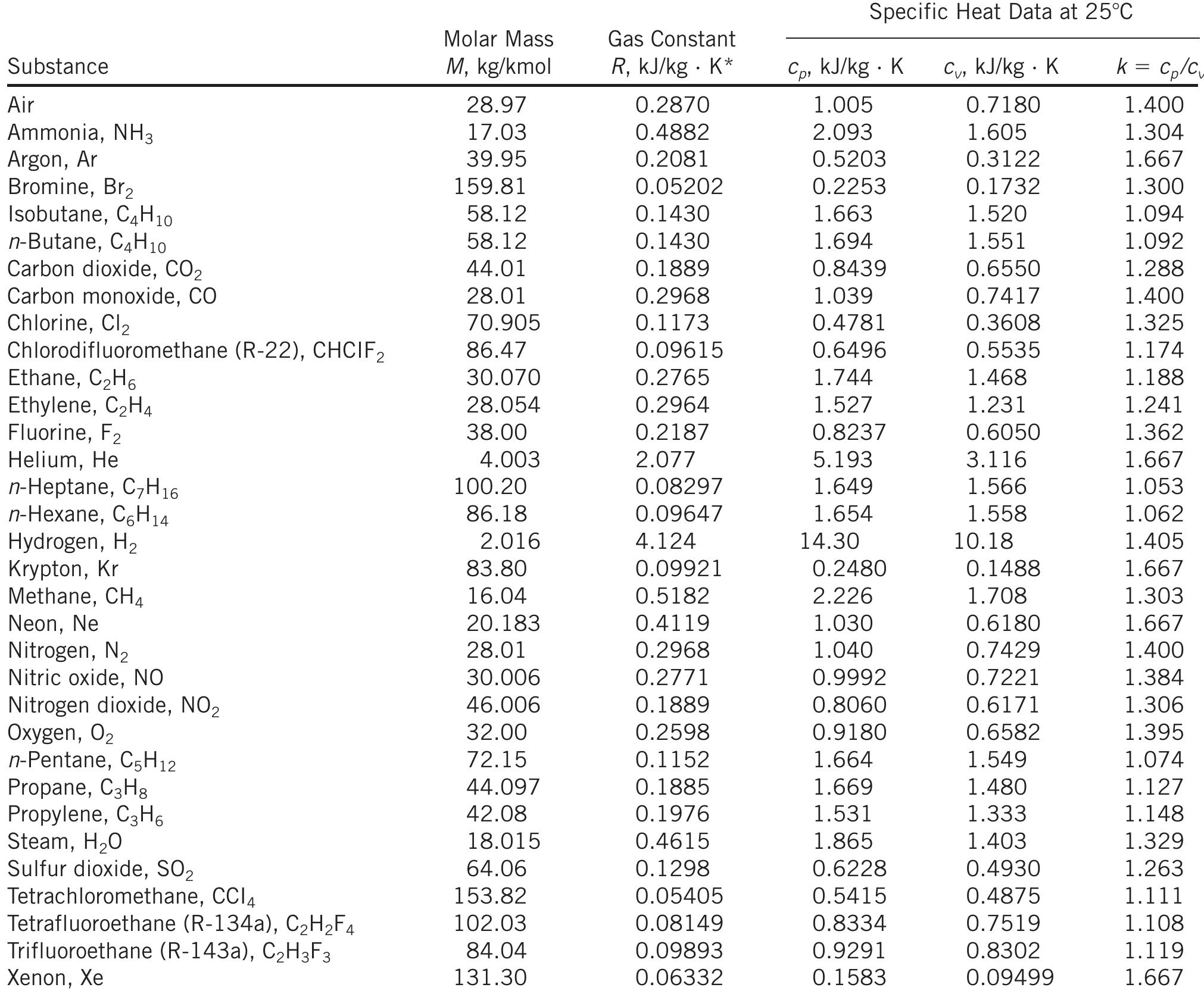
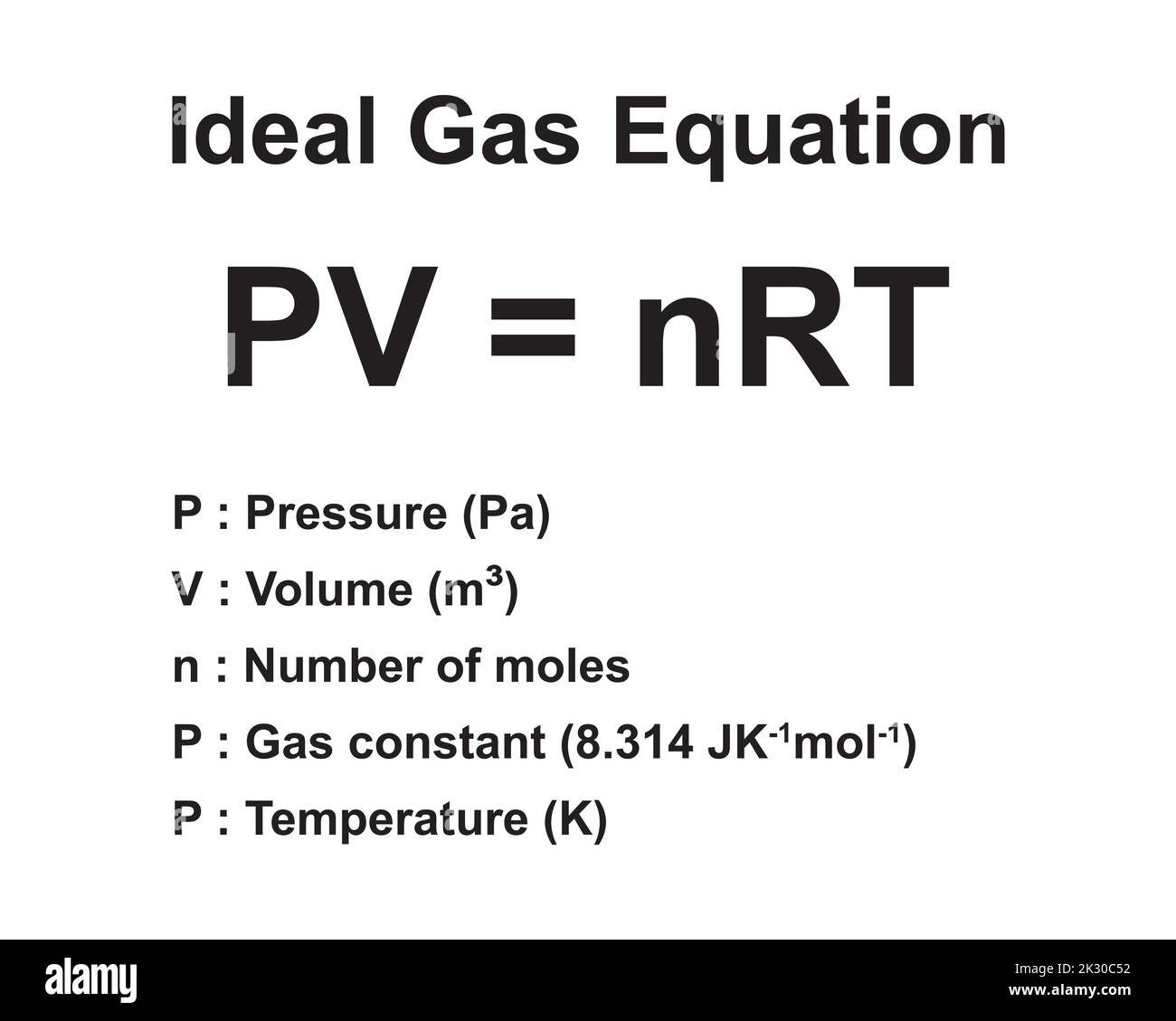
The ideal gas law—PV = nRT—serves as the cornerstone of gas analysis, where R represents the ideal gas constant. Unlike arbitrary scaling factors, IdealGasConstantR is rigorously defined: it sets the proportionality between the macroscopic state variables of a gas. Current standard value: IdealGasConstantR = 8.314 J/(mol·K), defined traceable to fundamental physical constants via the NIH precision scale.
This value emerges from precise measurements linking energy per mole to temperature in kelvins, ensuring consistency across scientific disciplines. When expressed in SI units, IdealGasConstantR permits direct conversion between energy changes and volume shifts efficiently: ΔV = Δ(PV) / R, a formula indispensable in fields ranging from HVAC systems to high-energy plasma research. The elegance of IdealGasConstantR lies not only in its numerical precision but in its universal applicability—applying equally to air at room temperature, combustion exhaust, or supercritical fluids under extreme pressures.
Though based on the idealized assumption of non-interacting, point particles, IdealGasConstantR remains remarkably accurate for many operational conditions.
Real gases approximate ideal behavior within a wide range—especially at moderate pressures and high temperatures—making R the go-to parameter in engineering thermodynamics tables and CAD simulations. Its precise calibration enables accurate modeling of critical processes like gas turbine efficiency and carbon capture operations.
From Lab Bench to Global Systems: Key Applications of IdealGasConstantR
In atmospheric science, IdealGasConstantR powers weather prediction models. By quantifying how temperature fluctuations affect atmospheric pressure and volume, meteorologists rely on R to interpret data from satellites and ground stations.
For instance, rising temperatures cause gas expansion; using R, algorithms calculate pressure drops at changing altitudes, enhancing forecast precision. Example: A sudden warm front increasing temperature by 10 K at sea level shifts air density by measurable margins—enabling early storm detection.
Industrial systems leverage IdealGasConstantR for safety and efficiency. In natural gas pipelines, pressure regulation depends on understanding how cooler nighttime conditions contract gas volumes, reducing risk of over-pressurization.
Power plants use R to model steam expansion in turbines, optimizing energy output and minimizing waste heat. Case study: Modern liquefaction plants exploit R’s predictive power to manage cryogenic temperatures, where near-ideal behavior enables efficient phase transitions—critical in LNG production and scientific sample preservation.
In aerospace, IdealGasConstantR underpins life support and propulsion. Spacecraft cabin systems use it to balance oxygen delivery with pressure stability, ensuring crew safety during extreme vacuum exposure.
Rocket nozzles rely on R to simulate exhaust gas dynamics, predicting thrust and velocity under varying ambient pressures—essential for mission success. Insight: Different gases (oxygen, hydrogen, helium) each follow the same ideal gas law, allowing universal modeling across propulsion chemistries, simplifying complex system design.
Precision and Limitations: When IdealGasConstantR Meets Reality
While IdealGasConstantR is a robust tool, real-world gases deviate from ideal behavior under extreme conditions—high pressures, low temperatures, or high densities. Water vapor, polar molecules, or reactive gases exhibit intermolecular forces and volume constraints that R alone cannot fully capture.
However, corrections using real gas equations (van der Waals, Redlich-Kwong) are built upon the ideal framework enabled by R, extending applicability without sacrificing foundational clarity. Scientific consensus: In most engineering contexts, IdealGasConstantR’s predictive capability remains unmatched, requiring only awareness of its limits in precision-sensitive domains like high-vacuum physics or supercritical fluid processing.
The calibration of IdealGasConstantR also reflects evolving metrology standards. Redetermined multiple times via particle physics and electrical resistance baths, its stability ensures consistency in global research and industry.
For instance, R’s value anchors the kelvin in the International System of Units, linking thermal energy directly to mole-based gas properties—a vital bridge between microscopic energy and macroscopic observables.
The Enduring Legacy of IdealGasConstantR in Modern Science
The Ideal Gas Constant, IdealGasConstantR, stands as a silent sentinel in modern science—its precision enabling accurate modeling across disciplines from environmental monitoring to interplanetary travel. More than a mere number, it embodies the fusion of theoretical physics and practical engineering, translating abstract gas laws into actionable insights. As computational power and detection technologies advance, IdealGasConstantR continues to serve as a foundational constant, ensuring that every calculation—from classroom demonstrations to spacecraft simulations—rests on a bedrock of consistency and reliability.
In the ever-evolving landscape of scientific discovery, IdealGasConstantR’s unassuming role proves indispensable: the heartbeat of gas behavior, the witness to pressure and volume, and the stealth enabler behind countless technological triumphs. Through its measured value, science gains a key to understanding the invisible forces shaping our world.
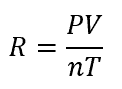

Related Post
Skye Wheatley leaves followers baffled thanks to a bizarre typo in her Instagram caption