Unlocking the Quantum Code: How Planck’s Constant in Electron Volts Defines the Scale of Energy at the Atomic Level
Unlocking the Quantum Code: How Planck’s Constant in Electron Volts Defines the Scale of Energy at the Atomic Level
Planck’s constant, encoded in electron volts, stands as the cornerstone of quantum physics—quantifying the discrete nature of energy at the smallest scales. Measured as ℎ/(2π) ≈ 4.135667696×10⁻¹⁵ eV·s, this fundamental constant transforms abstract quantum behavior into measurable, usable units that underpin modern technology, spectroscopy, and fundamental research. Every electron volt, a unit of energy equivalent to the kinetic energy an electron gains accelerating across 1 volt, gains profound meaning through its precise connection to Planck’s constant.
“The electron volt is not merely a unit—it is the bridge between quantum theory and engineering reality,” explains Dr. Elena Rostova, quantum metrology expert at the National Institute of Standards and Technology. At its core, the relationship ℎ = hν—energy equal to Planck’s constant times frequency—reveals the quantized essence of electromagnetic energy.
When translated to electron volts, Planck’s constant enables direct comparison across atomic transitions, particle physics, and nanoscale device design. For instance, the energy required to ionize hydrogen—the threshold for electronic excitation—is precisely defined by 13.6 eV, a value rooted in this constant. This quantization means energy changes at the atomic level are not continuous but occur in discrete packets, a principle that fuels lasers, semiconductors, and quantum computing leverage.
The Electron Volt: Defining the Threshold of Quantum Energy
The electron volt, though rooted in classical voltage concepts, finds its true value in Planck’s constant and time. One eV corresponds to the energy an electron gains when accelerated through an electric potential difference of one volt—approximately 1.602×10⁻¹⁹ joules. Yet without Planck’s constant, linking this macroscopic unit to the quantum realm requires precision.The redefined SI system, established in 2019, fixes h at exactly 6.62607015×10⁻³⁵ kg·m²/s, enabling exact conversion between frequency and energy. “ℎ in eV transforms abstract photons and electrons into tangible, calculable energy levels,” notes Prof. Marcus Lin from the University of Oxford, a specialist in quantum optics.
This synthesis supports technologies ranging from photoelectric sensors to high-resolution electron microscopes. In photovoltaics, for example, understanding how much energy photons deliver hinges on ℎ conversion via electron volts—optimizing solar cell efficiency demands precise knowledge of this constant. Similarly, quantum dots and single-electron transistors rely on energy levels defined by quantum harmonic oscillators, where excitation energies scale directly with ℎ / precision encoded in eV.
Quantum Standards and the Metrological Revolution
The redefinition of the kilogram and second via Planck’s constant—alongside the fixed speed of light and unstable cesium atomic clock—ushered in a new era of metrology. The precision enabled by ℎ in eV manifestations now supports devices like ultra-stable lasers and quantum clocks, responsible for GPS accuracy and secure communications. “Before this redefinition, quantum energy measurements relied on indirect calibrations—now we have direct traceability through fundamental constants,” states Dr.Carla Menezes, metrology scientist at the Physikalisch-Technische Bundesanstalt. This shift has profound impacts: inter-laboratory comparisons of energy measurements become globally consistent, reducing uncertainty in material science and chemical analysis. Opto-electronic components, essential in fiber optics and quantum information processing, are now tuned using quantum energy benchmarks defined by eV and ℎ.
The National Institute of Standards and Technology’s work exemplifies this: cryogenic single-photon detectors calibrated with spectral lines tied directly to quantum energy levels enhance sensitivity and reliability.
From Lab Bench to Life: Real-World Applications of Planck’s Constant in eV
Beyond laboratories, the influence of Planck’s constant in electron volts shapes daily life. In medical imaging, positron emission tomography (PET) scanners detect gamma photons—energies measured in MeV, million electron volts—derived from nuclear transitions calibrated through quantum theory.“Each photon detection hinges on understanding energy tagged by ℎ in eV,” clarifies Dr. James Tran, a radiophysicist at Stanford Medical Center. In semiconductor design, bandgap energies—critical for transistor operation—are quantified in eV, guiding the development of faster, smaller chips.
Modern electronics depend on precise control of electron excitation across energy bands, a process grounded in quantum mechanics and reliable through the ℎ link. Quantum teleportation experiments and quantum cryptography also operate at these energy thresholds, where trustworthy transmission hinges on measured, standardized electron volt values. Technological milestones, from quantum computing architectures to lunar rover instruments, are validated and optimized through energy calibration rooted in ℎ × eV.
The escalating miniaturization and power efficiency of devices reflect this quantum foundation, now invisible yet indispensable to innovation.
Planck’s constant, when expressed through electron volts, is far more than a numerical value—it is the quantum yardstick that shapes how energy is measured, manipulated, and understood across science and technology. From foundational physics to cutting-edge engineering, this constant bridges theory and practice, enabling precision-scale applications that define the modern era.
Its enduring relevance underscores a timeless truth: the smallest units of energy hold the keys to the largest technological revolutions.

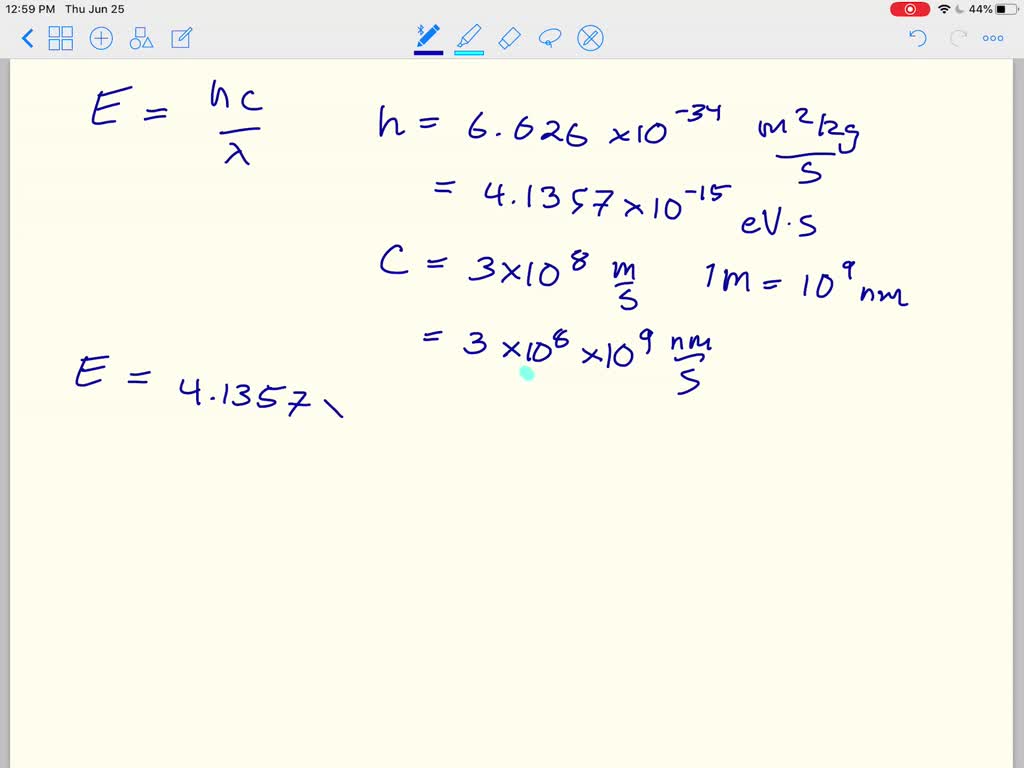


Related Post
Timothy Treadwell’s Voice From the Wild: A Sonic Journey into the Heart of the American West
Unveiling the Stature: Robert Redford Height, A Comprehensive Overview Of His Life, Career, and Enduring Legacy

Call 1-888-778-1868 to Unlock Instant Support for Critical Health and Safety Issues

Unlock the Soul of Indonesian Soul Music: Decoding “Sampe Tuwek” from Denny Caknan’s Lyrics and YouTube Legacy

