Unlocking the Mystery of Hydrogen Cyanide: Decoding Its Lewis Structure and Reactive Nature
Unlocking the Mystery of Hydrogen Cyanide: Decoding Its Lewis Structure and Reactive Nature
Hydrogen cyanide (HCN) is a compound shrouded in scientific intrigue—flashy in its chemical behavior, elusive in its molecular architecture. Central to its reactivity and utility is its precise Lewis structure, which reveals a simple yet remarkably balanced electron distribution. This article delves deep into the Lewis structure of hydrogen cyanide, unpacking its geometry, bonding, charge distribution, and significance in chemistry and industry—all while highlighting why its structure continues to captivate researchers and industrial chemists alike.
The Foundational Structure: Hydrogen Cyanide’s Simplest Lewis Form
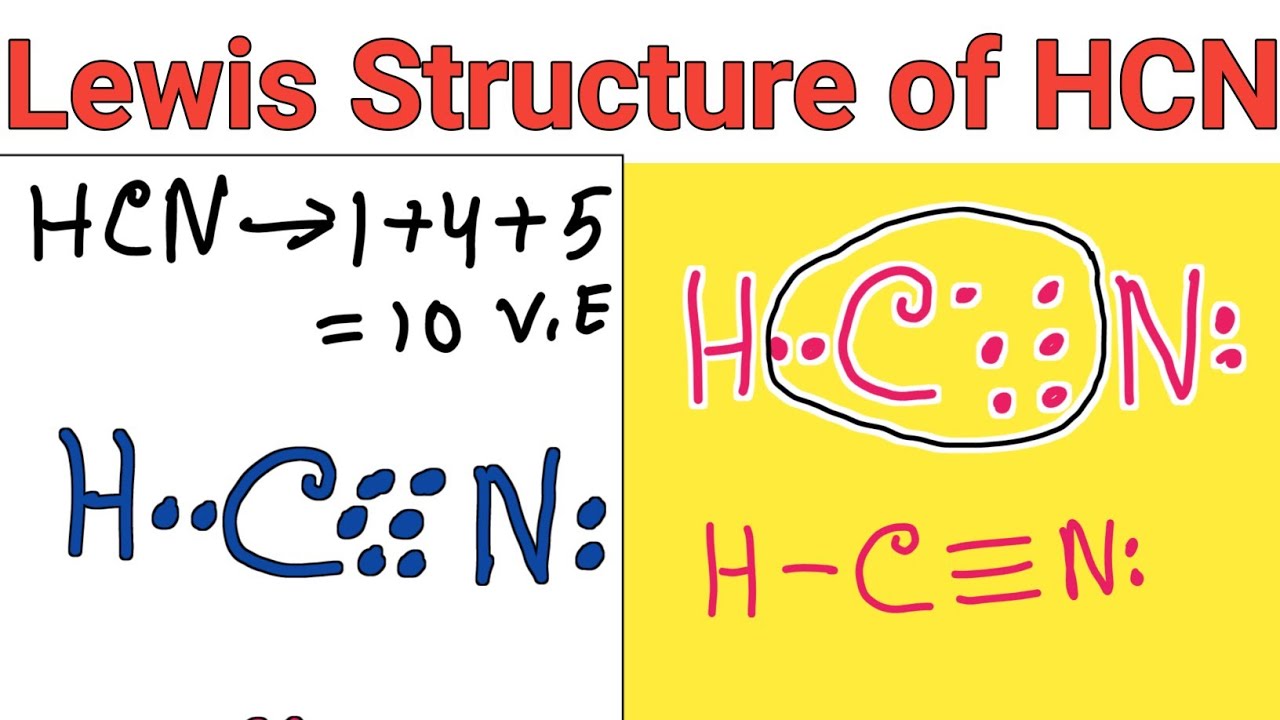
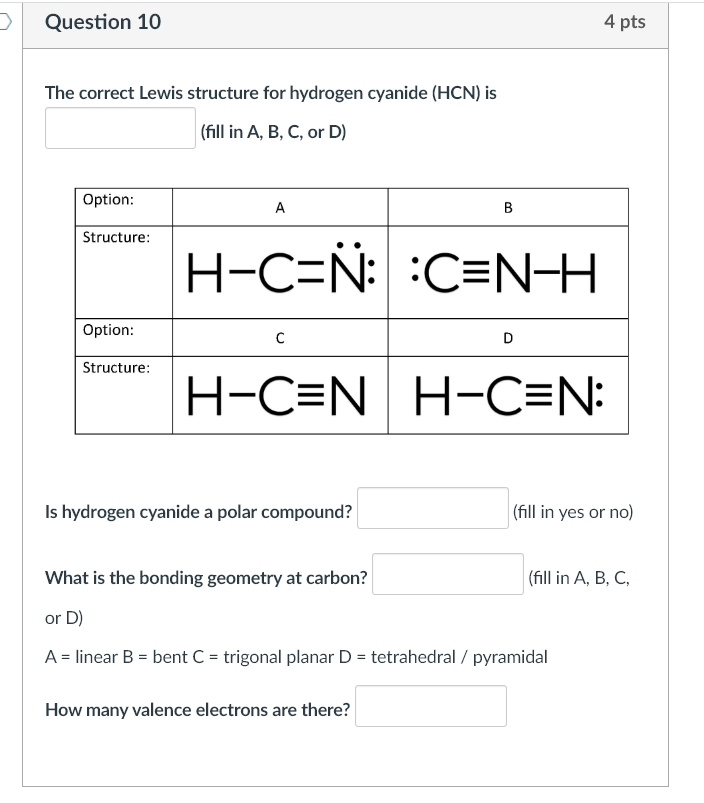
The Lewis structure of hydrogen cyanide presents a striking picture: one hydrogen atom covalently bonded to a nitrogen atom, which in turn shares a triple bond with a carbon atom, directly attached to a cyano group (-CN). Unlike many organic molecules with complex hybridization, HCN maintains a linear geometry due to the sp hybridization of the central carbon. This hybridization arises from the carbon’s two bonding electron pairs—one to hydrogen and one to nitrogen—resulting in a 180° bond angle.Chemists represent the molecular arrangement as H–C≡N, where the carbon achieves full octet participation through this triple bond. The hydrogen shares a single covalent bond:
H
C≡N
This elegant arrangement underscores HCN’s classification as a polar, reactive molecule despite its small molecular size.
Bonus fact: The triple bond in HCN spans approximately 10.7 picometers (pm), reflecting the high bond strength typical of triple bonds—over 800 kJ/mol—contributing to its stability under controlled conditions but reactivity under others.
Electron Distribution and Bond Polarity: The Key to Reactivity While the triple bond dominates HCN’s bonding profile, electronegativity differences shape the molecule’s polar character. Nitrogen (electronegativity: 3.04) is significantly more electronegative than hydrogen (2.20) and carbon (2.55), resulting in polar covalent bonds.
The electron density clusters near nitrogen, imparting a partial negative charge (δ⁻) to the terminal carbon, and a partial positive charge (δ⁺) on hydrogen and nitrogen. This polarity drives HCN’s common behavior as a weak acid in aqueous solution—despite the cyanide ion (-CN⁻) being a strong base. The molecule readily dissociates to release hydrogen ions, especially when catalyzed or exposed to basic environments.
H–C≡N → H⁺ + CN⁻
This dissociation is central to HCN’s utility in synthetic chemistry, where the cyanide anion serves as a versatile nucleophile and precursor to amines, nitriles, and polymers.Hybridization and Geometry: Why HCN Stays Linear Carbon’s sp hybridization is not just a geometric quirk—it fundamentally influences HCN’s electronic and physical properties. In sp hybridization, one 2s and one 2p orbital mix to form two equal-energy hybrid orbitals oriented 180° apart. This linear geometry constrains free electron rotation and limits conformational flexibility, enhancing the molecule’s stability.
Despite this rigidity, the molecule remains dynamic at the electron level: the triple bond restricts rotation freely, yet the lone pair on nitrogen permits rotation—confirming that molecular geometry and electronic behavior are distinct but interrelated. This duality allows HCN to maintain structural integrity while enabling selective reactivity.
Charge Distribution and Its Role in Chemical Interactions
The uneven charge distribution in HCN’s Lewis structure amplifies its chemical reactivity.The electrophilic hydrogen and labile cyanide anion create multiple reaction hotspots. Nucleophiles attack the δ⁺ carbon, while bases accept its proton—making HCN a cornerstone reagent in alkyl cyanation, hydrogen cyanide polymerization, and pharmaceutical precursor synthesis.
The cyanide ion (-CN⁻), formed upon deprotonation, is especially vital.
Its resonance-stabilized structure allows it to function as a weak ligand in metal complexes and as a key intermediate in cyanide-based detoxification reagents.
Safety and Toxicity: A Structural Perspective
The same structural features that enable HCN’s synthetic utility—particularly the highly polarized triple bond—also underlie its extreme toxicity.Cyanide’s ability to bind strongly to cytochrome c oxidase, disrupting cellular respiration, originates in the electron-rich nature of the cyanide ion. Despite HCN’s low volatility, its hydrogen lung penetration and slow but relentless cellular assault make it one of the most hazardous small molecules. Yet, in controlled industrial settings, understanding HCN’s Lewis structure allows chemists to design safer handling protocols—ventilation systems calibrated to capture traces, absorbent materials that bind dissolved HCN, and neutralization strategies that exploit bond cleavage.
The structure, therefore, is not merely academic: it is integral to risk assessment and mitigation.
The Enduring Significance of Hydrogen Cyanide in Science and Industry
Hydrogen cyanide’s Lewis structure—sparing yet sophisticated—epitomizes how simplicity in molecular architecture can yield disproportionate chemical power. From guiding electron flow to dictating reactivity, nitrile bonds are foundational to modern synthetic pathways.The linear, sp-hybridized geometry, balanced polarity, and strategic charge distribution make HCN both a laboratory workhorse and an industrial linchpin. As researchers explore greener synthesis routes and new materials, the Lewis structure of HCN remains a vital reference point—illuminating the molecular logic behind transformations that shape medicines, polymers, and chemical innovation. Its story, written in lines of electrons and bonds, continues to unfold with impact far beyond the laboratory bench.


Related Post

416 Area Code: Canada’s Timeless Symbol of Telephone Identity and Community Heritage
Saturn’s Seasonal Journey: A Deep Dive into Its Distance From the Sun
Detailing the Journey of Ella Bailey Saucedo: A Fresh Phenom

