Unlocking Nature’s Power: How Exergonic Chemical Reactions Drive Energy Release
Unlocking Nature’s Power: How Exergonic Chemical Reactions Drive Energy Release
Some of the most fundamental processes powering both life and technology originate in a class of chemical reactions defined by energy release: exergonic chemical reactions. Far more than abstract chemistry concepts, these reactions underpin everything from cellular respiration to industrial power generation. Understanding their mechanisms and real-world applications reveals the quiet engine behind countless natural and engineered systems.
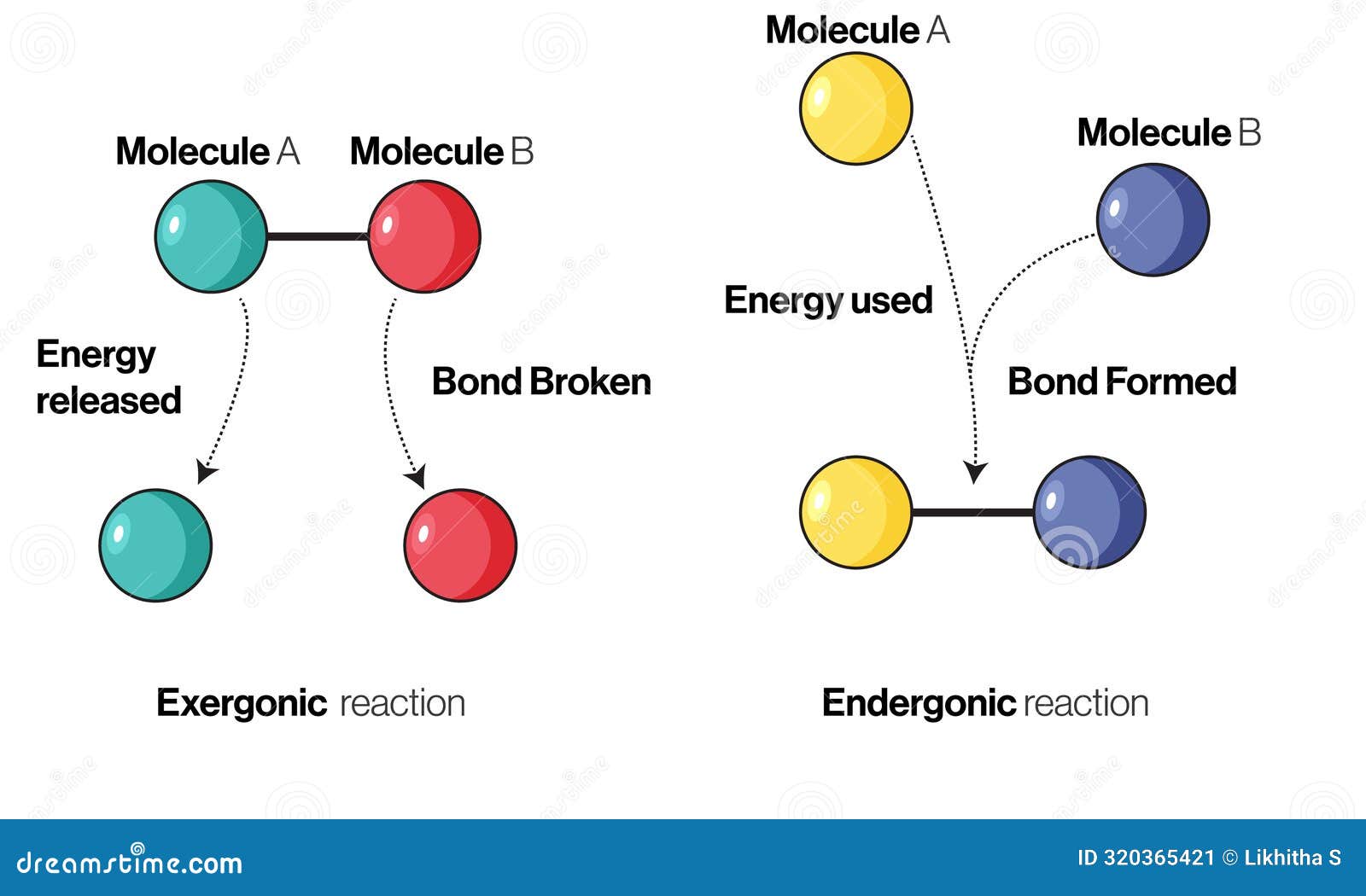
At the core, an exergonic reaction is one in which the total free energy of the system decreases, releasing usable energy—typically as heat or mechanical work—into the surroundings. The difference between the initial and final free energy states defines the reaction’s enthalpy change, measured in kilojoules per mole (kJ/mol). When this change is negative (ΔG < 0), the reaction proceeds spontaneously without external energy input.
“Exergonic processes convert stored chemical energy into functional forms like ATP, kinetic energy, or thermal output with remarkable efficiency,” explains Dr. Elena Marquez, a physical chemist at ETH Zurich. “They represent nature’s most reliable energy transduction mechanisms.”
The most ubiquitous example lies at the heart of biological metabolism: cellular respiration.
In this process, glucose undergoes oxidation through a series of exergonic reactions, culminating in the respiration of oxygen and production of carbon dioxide, water, and energy. The energy released drives the synthesis of adenosine triphosphate (ATP)—cellular fuel—enabling movement, biosynthesis, and nerve signaling. According to the National Institutes of Health (NIH), this exergonic cascade derives a net energy yield of approximately -2870 kJ/mol for complete glucose oxidation.
Key Features of Exergonic Reactions:- Negative Gibbs Free Energy (ΔG): The system releases free energy, enabling work to be performed.
- Spontaneous Progression: These reactions occur naturally under standard conditions without ongoing external input.
- Energy Release:** Energy is predominantly emitted as heat or converted into favorable bond formation.
- Common in Redox and Acid-Base Processes: Combustion, cellular metabolism, and neutralization reactions exemplify this classification.
Beyond biology, exergonic chemical reactions are pivotal in energy technology.
Fuel cells, essential for clean energy transport, operate on exergonic oxidation of hydrogen and oxygen to form water and electricity—with efficiency surpassing traditional combustion engines. The hydrogen fuel cell, for instance, converts chemical energy directly into electrical energy through a controlled exergonic process, emitting only water vapor and heat.
Two widely studied exergonic pathways illustrate this phenomenon:
- Combustion Reactions: Rapid oxidation of fuels like methane or gasoline releases vast energy. Methane’s combustion:
- Redox Reactions in Bioenergetics: In mitochondria, electron transport chains drive proton gradients through exergonic redox steps, powering ATP synthase. These cascades exemplify nature’s precision in energy harnessing.
While exergonic reactions release energy, their rate and control depend on activation barriers. Catalysts, such as enzymes in cells or platinum in fuel cells, lower energy thresholds to initiate the reaction safely and efficiently.
Applications extending beyond biology include industrial synthesis, where exergonic reactions supply heat for chemical manufacturing, and electrochemical systems like batteries, where controlled exergonic discharge powers portable devices.
Each relies on the fundamental principle that energy released under favorable thermodynamic conditions can be captured and directed to perform work.
Real-World Impact and Future Frontiers Example: Hydrogen Economy The transition to hydrogen as a clean fuel depends entirely on managing exergonic oxidation. Efficient catalysts and hydrogen storage systems aim to unlock maximum energy yield with minimal waste, promising a low-carbon future. Challenges remain, particularly in preventing energy loss and ensuring storage safety—but progress continues to hinge on deepening understanding of exergonic reaction dynamics.Environmental and Economic Drivers Exergonic processes offer a blueprint for sustainable energy: transforming stored chemical energy into clean, usable forms with reduced emissions. As global energy demand rises, optimizing these reactions delivers both efficiency and environmental benefit. “Exergonic chemistry is not just central to science—it’s key to solving climate challenges,” asserts Dr.
Marquez.
At their core, exergonic chemical reactions reveal nature’s elegant design: converting stored energy into functional output with precision and minimal entropy increase. From the mitochondria to hydrogen fuel cells, these reactions not only power life but enable human innovation.
By mastering the principles of energy release through spontaneous chemical change, science advances toward a future where clean, efficient energy flows seamlessly from molecular interactions to the world we powers.



CH₄ + 2O₂ → CO₂ + 2H₂O + 890 kJ/mol “This single reaction supplies more than 50% of global electricity in gas-fired power plants,” notes energy researcher Dr.
James Lin from MIT’s Energy Initiative.
Related Post

Unraveling the Mystery of DTI Prisoner or Cop: When Authority Faces Illusion

Jayson Tatum’s Stature: A 6’8” Basketball Giant Redefining Size and Impact on the Court
Sanrina Carpenter: The Rising Star Reimagining Pop Through Authentic Artistry

