Unlocking Mixture Mysteries: How Mole Fraction Formula Powers Chemistry Design
Unlocking Mixture Mysteries: How Mole Fraction Formula Powers Chemistry Design
In the invisible world of chemical mixtures, precision is everything—and nowhere is this clearer than in the application of the mole fraction formula. For scientists, engineers, and researchers, mole fraction serves as a fundamental metric that quantifies the relative composition of components within a blend, enabling accurate predictions of physical properties and reaction behaviors. Mastering this concept is not just academic—it’s essential for designing processes, optimizing industrial production, and advancing material science.
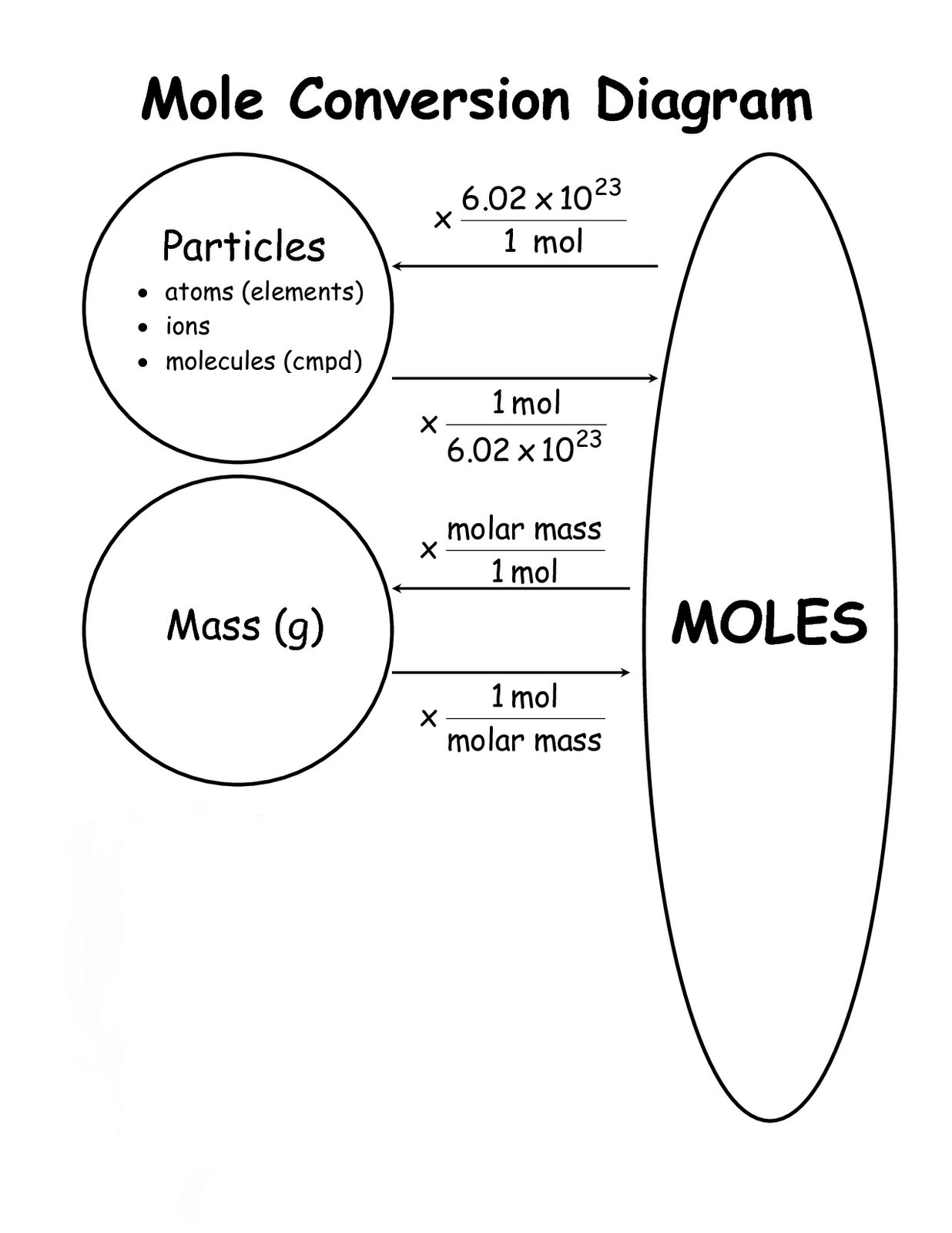
The mole fraction, defined as the ratio of the number of moles of a component to the total number of moles in the mixture, is expressed mathematically as: **x_i = n_i / Σn_j** where *x_i* is the mole fraction of component *i*, *n_i* is the moles of species *i*, and the summation runs across all components in the system. This elegant formula provides a dimensionless, universal measure that remains valid regardless of temperature or pressure—critical for reproducibility across labs and industries.
At its core, mole fraction bridges microscopic composition with macroscopic observables.
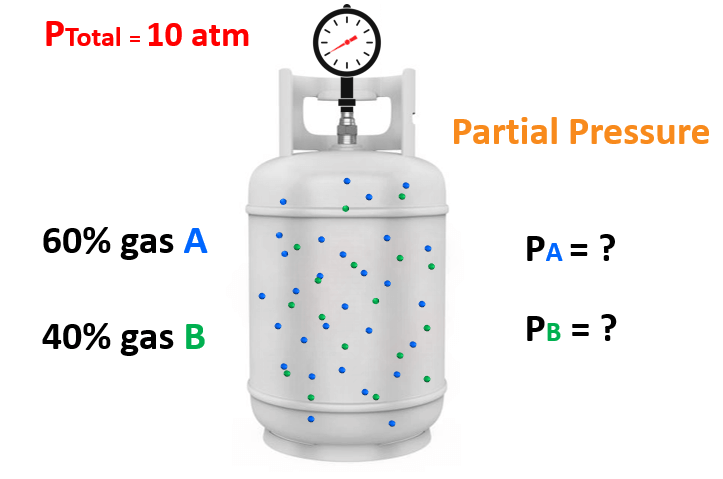
For instance, in gas mixtures, mole fraction directly correlates with partial pressures through Dalton’s Law, allowing engineers to calculate how each gas contributes to total system pressure. More than a mere ratio, it underpins thermodynamic modeling, phase equilibrium analysis, and solution behavior predictions.
Core Components of the Mole Fraction Concept
Understanding mole fraction requires unpacking its three essential elements: mole, component, and total moles. A mole, the SI unit of amount of substance, represents 6.022 × 10²³ elementary entities—atoms, molecules, or ions—ensuring consistency across chemical systems.A component is any discrete chemical species within the mixture—such as water in ethylene glycol or oxygen in air. The total moles are the sum of all individual moles, forming the normalization factor in the formula. The formula’s simplicity belies its power: it transforms qualitative descriptions of mixtures—“mostly water with traces of alcohol”—into precise, quantitative data.
This transition enables accurate calculations of defect annealing in semiconductors, vapor pressure reduction in pharmaceutical formulations, and efficiency of catalytic reactions.
Consider a binary mixture of methane and nitrogen. If a sample contains 2 moles of methane and 8 moles of nitrogen, the mole fraction of methane is: **xCH₄ = 2 / (2 + 8) = 0.20** Consequently, methane constitutes 20% of the mixture and occupies 20% of the total moles.
This direct proportionality simplifies modeling: furnace efficiency can be estimated using mole fraction to balance reactant flow, while diffusion rates depend explicitly on composition weights. <ü>Mole Fraction and Real-World Applications The utility of mole fraction transcends theoretical chemistry, embedding itself in industrial practice. In petroleum refining, distillation columns rely on mole fraction data to separate crude oil into fractions like gasoline, diesel, and kerosene.
Each stream’s composition—defined precisely by mole fractions—dictates energy input, equipment design, and output quality. Similarly, in carbon capture technologies, mole fraction determines CO₂ retention efficiency in solvent mixtures, enabling cost-effective emissions reduction. In pharmaceutical development, drug formulation hinges on mole fraction control.
A drug solution’s activity depends not just on concentration, but on how individual component moles interact—affecting solubility, stability, and bioavailability. Here, mole fraction guides precise dosing and regulatory compliance.
Environmental science also leverages this formula.
Atmospheric studies quantify greenhouse gas contributions using mole fractions, translating sensor data into global impact metrics. Urban air quality indices integrate mole fraction measurements to assess pollutant concentration relative to ambient air, supporting public health policies and emission regulations. Challenges and Precision in Measurement Despite its elegance, accurate mole fraction calculation demands rigorous experimental design.
Errors in mole determination—such as misidentifying stoichiometry or failing to account isotopic variants—distort results. Advanced techniques like molar gas analysis via infrared spectroscopy, mass spectrometry, or precise gravimetric analysis ensure reliable input values, minimizing uncertainty in derived fractions. Moreover, mole fraction’s invariance to phase changes supports modeling across gas-liquid-solid intervals—critical in multiphase reaction engineering and geochemical systems.
Mole Fraction in Complex Systems: Mixtures Beyond Ideal Behavior While the basic formula assumes ideal mixing, real-world applications often confront non-ideal behavior, where molecular interactions alter vapor pressure and diffusion. Yet even in these cases, mole fraction remains the foundational reference point. Activity coefficients and fugacity corrections are built upon mole fraction to adjust predictions, preserving the formula’s central role.
For example, in liquid-liquid extraction used to purify rare earth elements, accurate mole fraction data of solutes enables optimization of solvent systems, maximizing recovery while minimizing reagent use. Here, variability in intermolecular forces demands refined modeling—but always anchored by mole fraction baselines.
As computational chemistry evolves, mole fraction continues to underpin simulations and machine learning models predicting mixture properties.
Its enduring relevance stems from simplicity, universality, and robustness across scales—from lab-scale reactors to planetary atmospheres.
Mole fraction is far more than a mathematical expression; it is the silent architecture of mixture science. From refining fuels to designing life-saving drugs, from balancing industrial processes to safeguarding the environment, its precise application empowers innovation and accuracy. Understanding and leveraging the mole fraction formula equips professionals to navigate complexity with confidence, transforming chaotic blends into predictable, controllable systems.In a world defined by chemical interactions, mastering this formula is not optional—it is indispensable.
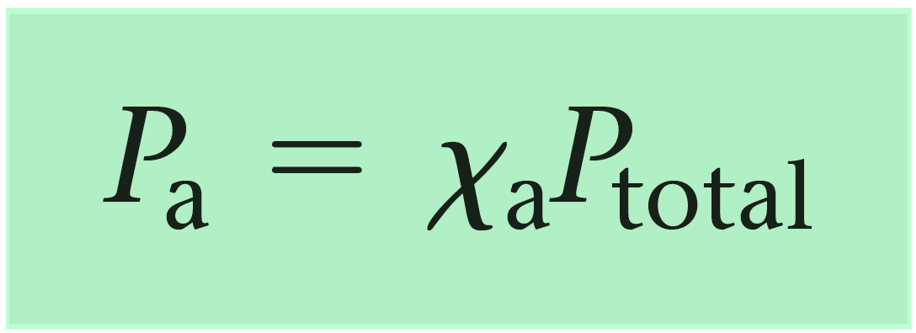
Related Post

The Legal Lifeline: How the Atlanta Volunteer Lawyers Foundation Empowers Underserved Communities