Unlocking Life’s Energy: The Power Behind Cellular Respiration and Its Critical Chemical Equation
Unlocking Life’s Energy: The Power Behind Cellular Respiration and Its Critical Chemical Equation
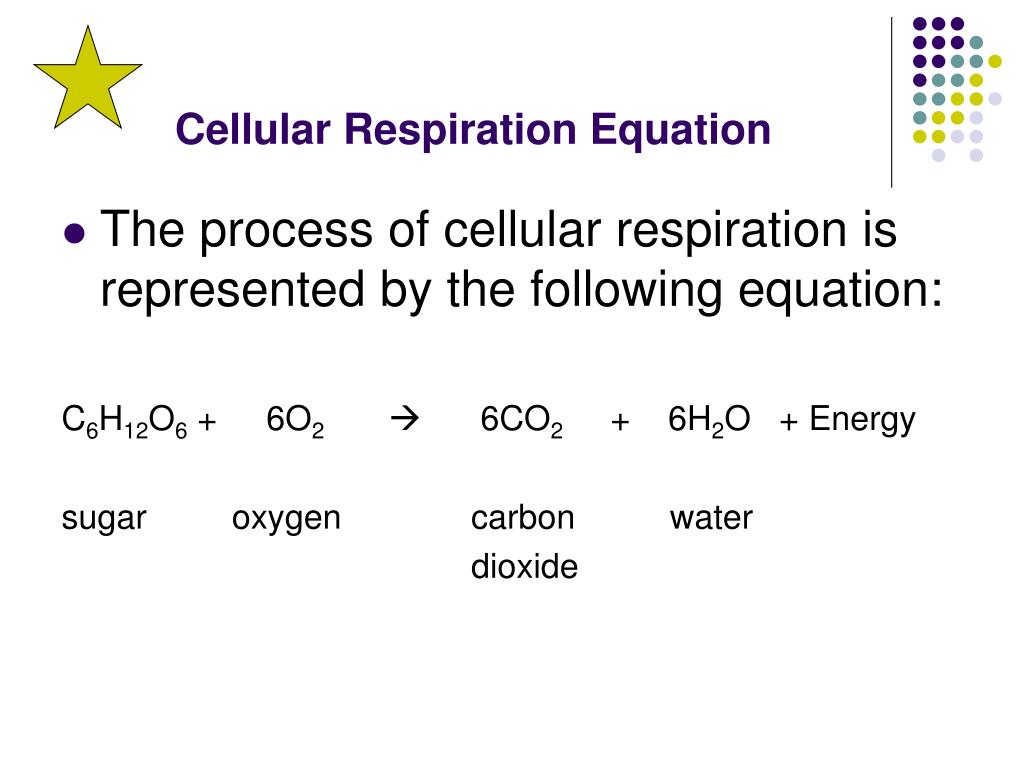
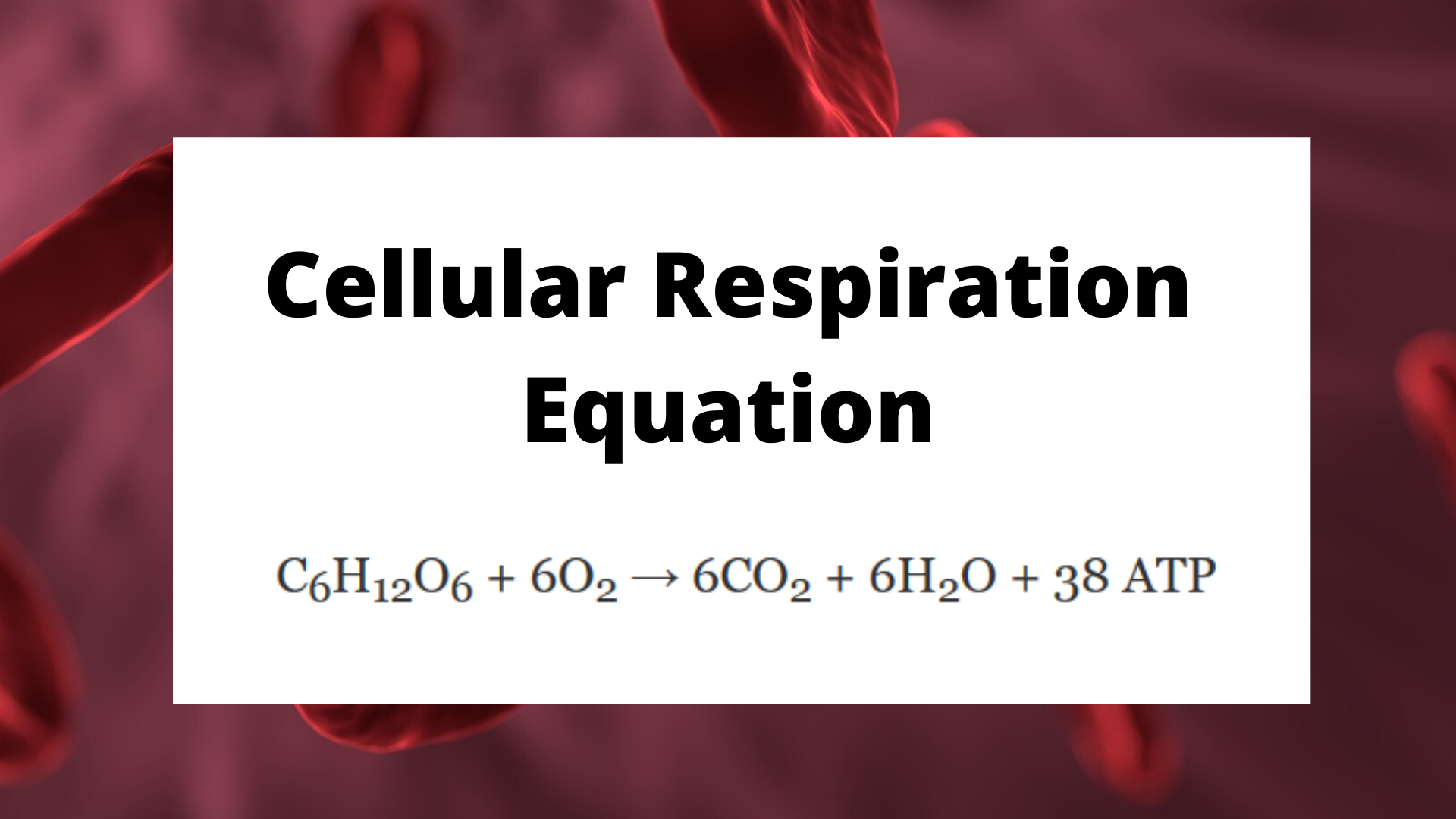
At the heart of every living cell lies an intricate molecular engine—the process of cellular respiration—which transforms stored chemical energy into the universal fuel of life: adenosine triphosphate (ATP). This biochemical powerhouse, powered by the precise rearrangement of carbon, hydrogen, and oxygen atoms, is encapsulated in a single yet profoundly significant equation: C₆H₁₂O₆ + 6O₂ → 6CO₂ + 6H₂O + ~38 ATP. This equation sums centuries of scientific discovery into a concise form, representing the oxidation of glucose in the presence of oxygen to yield carbon dioxide, water, and the energy currency of cells.
Far from being a mere formula, this reaction reveals how organisms convert nutrients into usable energy, sustaining growth, movement, and all vital functions. The core equation of cellular respiration—its chemical blueprint—can be broken down into distinct stages, each essential to extracting and delivering energy efficiently. The reactants, glucose and oxygen, initiate a cascade of redox reactions within cellular mitochondria: - **Glucose (C₆H₁₂O₆)** serves as the primary fuel, a six-carbon sugar abundant in carbohydrates, the most common dietary energy source.
- **Oxygen (O₂)** acts as the terminal electron acceptor, crucial for maximizing ATP production through oxidative phosphorylation. - The kinetic energy stored in glucose bonds is released through a series of enzymatic transformations, driving the synthesis of ATP via substrate-level phosphorylation and the electron transport chain. Standing at the center of this energy transformation is the equation’s right-hand side, where glucose and oxygen are converted into carbon dioxide, water, and a net yield of ATP—approximately 38 molecules per glucose molecule under optimal aerobic conditions.
While real cells may produce slightly fewer due to inefficiencies, this value remains a benchmark for aerobic respiration.
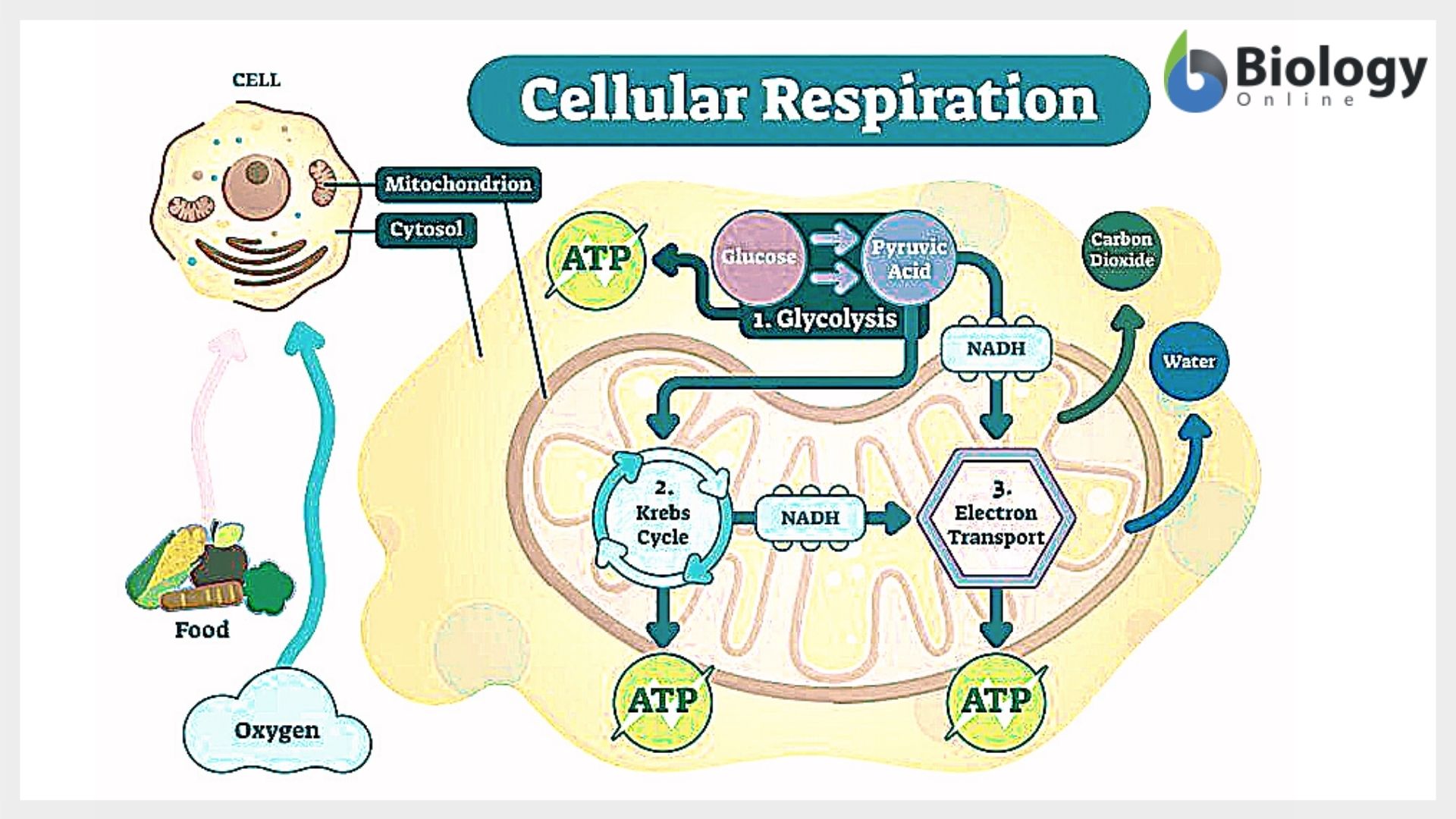
Recent studies confirm that this equation represents not just a sum of reactants and products, but a finely tuned sequence of biochemical pathways: glycolysis, pyruvate oxidation, the Krebs cycle, and oxidative phosphorylation. Each step relies on specific enzymes, coenzymes, and molecular carriers to shuttle electrons and protons across critical membranes.
“Cellular respiration is nature’s fuel processor,” explains biochemist Dr. Elena Marquez, “converting molecular bonds into energy with remarkable efficiency—where most metabolic processes excel not by perfection, but by precision.” This precision ensures that energy is neither wasted nor lost but stored in ATP’s high-energy phosphate bonds. The Krebs cycle, unfolding in mitochondrial matrix, regenerates electron carriers NADH and FADH₂—molecular relays packed with high-energy electrons. In the inner mitochondrial membrane, the electron transport chain spins protons down their electrochemical gradient, using the energy flux to drive ATP synthase, the molecular turbine producing most cellular ATP. The oxygen consumed at this stage combines with electrons and protons to form water—a byproduct that, while not energy-yielding, is vital for sustaining the proton flow that powers ATP synthesis. Despite its efficiency, cellular respiration operates under physical and biochemical constraints. The theoretical maximum of 38 ATPs per glucose hinges on ideal conditions, but real cells face losses due to proton leakage and coupling between electron flow and ATP production. “Biological systems are never 100% efficient,” notes Professor Rajiv Nanda, a molecular biologist specializing in energy metabolism. “Yet this near-optimal balance is why aerobic respiration dominates life forms from bacteria to humans.” Variations exist—anaerobic respiration produces far less ATP by using alternative electron acceptors like sulfate or nitrate—but aerobic respiration remains dominant in oxygen-rich environments due to its superior energy yield and rapid ATP generation. Glycolysis initiates the process in the cytoplasm, splitting glucose into two pyruvate molecules while generating a modest payoff of 2 ATP and 2 NADH—molecular carriers primed for oxidative processing. In oxygen-rich cells, pyruvate enters the mitochondria, where it is converted to acetyl-CoA, linking glycolysis to the Krebs cycle. This cycle regenerates electron carriers (NADH, FADH₂) and scavenge per cycle, producing two ATP via substrate-level phosphorylation. However, its greater significance lies in feeding high-energy electrons into the electron transport chain (ETC). Here, NADH and FADH₂ donate electrons across membrane-bound protein complexes—Complexes I to IV—each step releasing energy used to pump protons into the intermembrane space. The resulting electrochemical gradient, or proton-motive force, drives ATP synthase—a molecular turbine that converts proton flow back into ATP. This oxidative phosphorylation accounts for approximately 30 of the net 38 ATP molecules. Oxygen plays a silent but indispensable role: as the final electron acceptor, it combines with protons to form water, completing the redox reaction and maintaining electron flow. This dependency explains why aerobic organisms thrive in oxygen-rich environments, while anaerobic pathways—used by yeast and some bacteria—sustain life in oxygen-deprived niches with far lower efficiency. The equation’s conservation of mass and energy underscores its scientific rigor. Each oxygen atom absorbed binds with electrons to form water; each carbon atom in glucose exits as carbon dioxide. It’s not merely a consumption-and-waste model but a reconfiguration of matter that sustains cellular work. As biochemist Margaret Liu asserts, “Cellular respiration transforms food into a form usable by every enzyme, every membrane transport protein, every structure demanding energy—linking nutrition, thermodynamics, and life’s continuity in one seamless reaction.”The Biochemical Stages: Mapping the Pathway of Energy Transformation
Cellular respiration unfolds through three interconnected metabolic pathways, each responsible for distinct energy transformations.Biological Significance: Energy for Life’s Delicate Machinery
The output of cellular respiration—the ATP molecules—serves as the immediate energy source for countless cellular processes. Muscle contraction, active transport across membranes, DNA replication, and synaptic signaling in neurons all depend on ATP’s high-energy phosphate bonds. “ATP is the universal currency,” says neuroscientist Dr.
Carlos Mendez, “enabling the precision and speed required for life’s complexity. Without its regulated production, none of the machinery of life could operate.” Beyond energy, the equation’s byproducts play secondary roles. Carbon dioxide, exhaled in mammals, is a byproduct but also signals metabolic status—high levels trigger respiratory adjustments to maintain homeostasis.
Water, though a ‘waste’ product in this metabolic sense, supports cellular structure and serves as a reactant in some biosynthetic pathways. “Every cell balances efficiency and sustainability,” observes Dr. Nanda.
“The byproducts of respiration, though not energy-yielding, are vital for maintaining internal equilibrium.”
This metabolic elegance explains the universality of cellular respiration across domains of life. From fungi breaking down organic matter to neurons firing in a waking brain,

Related Post

Unveiling The Mystery of Shanin Blake’s Onlyfans Leak: What Happened, Why It Matters
SD Points: The Strategic Asset Reshaping Modern Military and Competitive Advantage
Matthew Hussey Youtube Bio Wiki Age Height Family Wife Podcast Books And Net Worth

