Unlocking Fluorine’s Chemical Identity: Decoding Its Lewis Structure to Master Reactivity
Unlocking Fluorine’s Chemical Identity: Decoding Its Lewis Structure to Master Reactivity
Fluorine, the most electronegative element on the periodic table, defies conventional expectations in chemistry—particularly when viewed through its Lewis dot structure. Despite being a halogen tightly bound in a tightly knit;iframe unit with only seven valence electrons, Fluorine’s dipole rarity and unique electron configuration shape its role as both a fierce reactant and a pivotal player in molecular architecture. This article unpacks the fluoride Lewis dot structure not just as a static diagram, but as a dynamic foundation for understanding Fluorine’s extreme chemistry—from bond distortion to nucleophilic dominance.
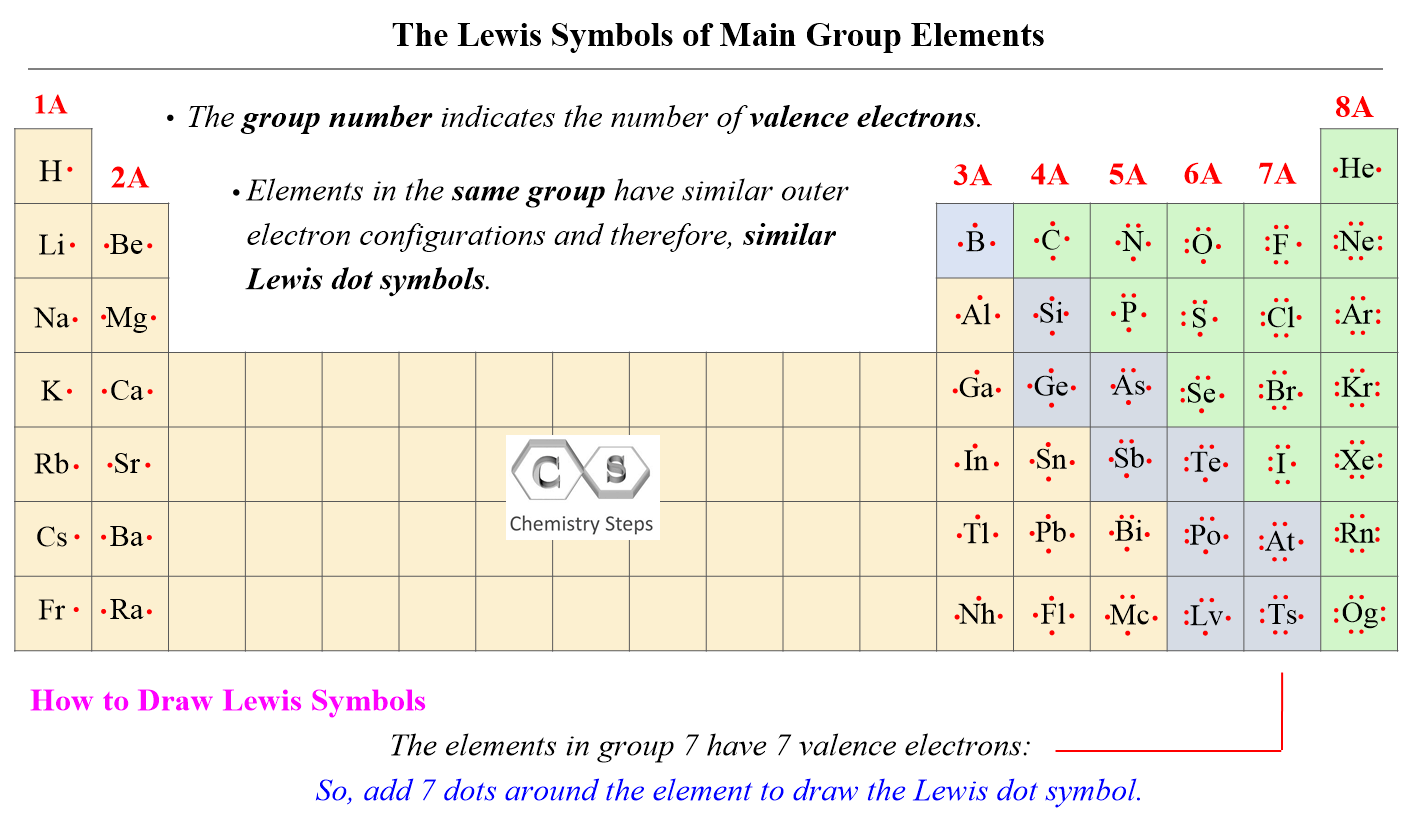
At its core, the Fluorine Lewis dot structure reveals a central fluorine atom surrounded by seven lone electrons and one untenanted p orbital empty or partially populated, hinting at profound chemical behavior. With seven valence electrons—three in each bonding pair and one lone pair—the structure aligns with the octet rule minimally, as Fluorine often prioritizes electronegativity over complete octet completion when interacting with metals or highly electronegative elements. Unlike typical second-period elements, Fluorine’s small atomic radius and strong effective nuclear charge create unique electron density patterns that influence molecular geometry and reactivity.
The Electron Arrangement: Core Features of Fluorine’s Lewis Structure
Swiftly analyzing the structure: - Fluorine occupies the center with seven valence electrons, displayed via dots outside a closed symbol.- In its pure form, Lewis structure often shows one single bond (two shared electrons) and five lone pairs, or alternatively, a structure with a lone pair and expanded interactions in certain complexes. - The central atom carries a formal positive charge when single-bonded, due to Fluorine’s ability to stabilize partial positive states via electron withdrawal. - Though Fluorine rarely expands its valence shell (being in period 2), in advanced compounds—especially with heavy metals—partial electron delocalization can alter expected electron counts.
A common representation places one single bond with five lone electrons around Fluorine, emphasizing its electron-deficient nature. Yet in hypervalent fluorides like K₂[F₅] or metal-fluoride complexes, transient charge separation and unshared electron pairs enable coordination that challenging traditional bonding models.
Bonding Dynamics: Electronegativity vs. Orbital Compatibility
Fluorine’s dominance in ionic and covalent bonding stems from its unmatched electronegativity—3.98 on the Pauling scale—leading to polar bond formation that nearly drains shared electrons.When bonding with a polyatomic ion like oxide (O²⁻), Fluorine typically forms a single bond while accepting electron density, pulling the joint electron pair toward itself. This results in a highly polar molecule, essential in biological and industrial processes.
In contrast, when Fluorine bonds with highly electronegative metals such as calcium or alkaline earth elements, strong electrostatic attraction stabilizes ionic lattices, yet the crystal structure reflects Fluorine’s role as a small, tightly held anion, influencing lattice energy and thermal stability.
Exceptional Electron Distribution and Structural Constraints
Though five lone pairs sound plausible, strict adherence to formal charge and minimal formal charge principles limits Fluorine’s lone electron inventory.In reality, Lewis structures prioritize resonance stabilization and charge minimalization. For example, in hydrogen fluoride (HF), the single bond and single lone pair match structural realism, while exotic forms such as [F₂]⁻ illustrate transient electron delocalization rare in elemental Fluorine. These rare configurations reveal Fluorine’s transient bond-breaking tendencies under external stimuli like light or catalysis.
Structural rigidity and low bond elongation—F–F bond length spans just 134 pm—reflect strong covalent character due to high electron density localization. This compact bond geometry minimizes steric strain and enhances reactivity in nucleophilic substitution, where Fluorine’s weak carbon–F fluorescence further amplifies its leaving group potential after activation.
Fluorine’s Reactivity: How Its Lewis Structure Dictates Behavior
Understanding Fluorine’s reactivity begins with its electron distribution. The high formal positive character at Fluorine, especially in hybrid environments, drives its role as the ultimate electrophile.With bonds designed for electron withdrawal, Fluorine readily facilitates nucleophilic attack—whether in overcoming fused-ring systems or activating substrates for cross-coupling.
In organic synthesis, Fluorine’s Lewis structure underscores why fluorination improves metabolic stability and bioavailability in pharmaceuticals. The electron-withdrawing effect alters ionization states, stabilizes reactive intermediates, and modulates molecular recognition.
Likewise, in materials science, Fluorine’s rigid;iframe structure underpins high-performance polymers, ion-exchange resins, and fluorinated coatings—each leveraging the atom’s distinct polarization capacity.
Applications Shaped by Valence Insights
From industrial atoms to antibiotic design, Fluorine’s electron architecture enables breakthroughs: - **Pharmaceuticals:** Introducing Fluorine atoms surfs metabolic degradation, extending drug half-life—ep
Related Post
Exposing the Dynamic Teamwork of James Roday With
<strong>Tnxp Stock Twits: Decoding Real-Time Market Sentiment That Shapes Today’s Trading Decisions</strong>

Share Internet Connection in Hyper-V: A Precision Guide to Secure, Efficient Network Integration
Taryn Dakha Was Romany Malcos Wife for 8 Years

