Unlocking Energy Changes: The Science and Significance of Reaction Enthalpies
Unlocking Energy Changes: The Science and Significance of Reaction Enthalpies
Every chemical reaction carries an invisible charge of heat—some releasing energy, others demanding it. Understanding reaction enthalpies, as explored in Learning Module 54, reveals how fundamental thermodynamics governs everything from industrial manufacturing to biological metabolism. This critical parameter, expressed in joules per mole, quantifies the heat absorbed or evolved during a chemical transformation, offering profound insights into reaction feasibility, energy efficiency, and process design.
The Thermodynamic Heartbeat of Chemical Reactions
At the core of chemical thermodynamics lies the concept of enthalpy—a measure of total heat content in a system at constant pressure.
Reaction enthalpy, denoted as ΔHrxn, defines the difference between the enthalpies of products and reactants: ΔHrxn = ΣΔHproducts − ΣΔHreactants. When ΔHrxn is negative, a reaction releases heat (exothermic); positive ΔH indicates endothermic transformations, requiring external energy input.
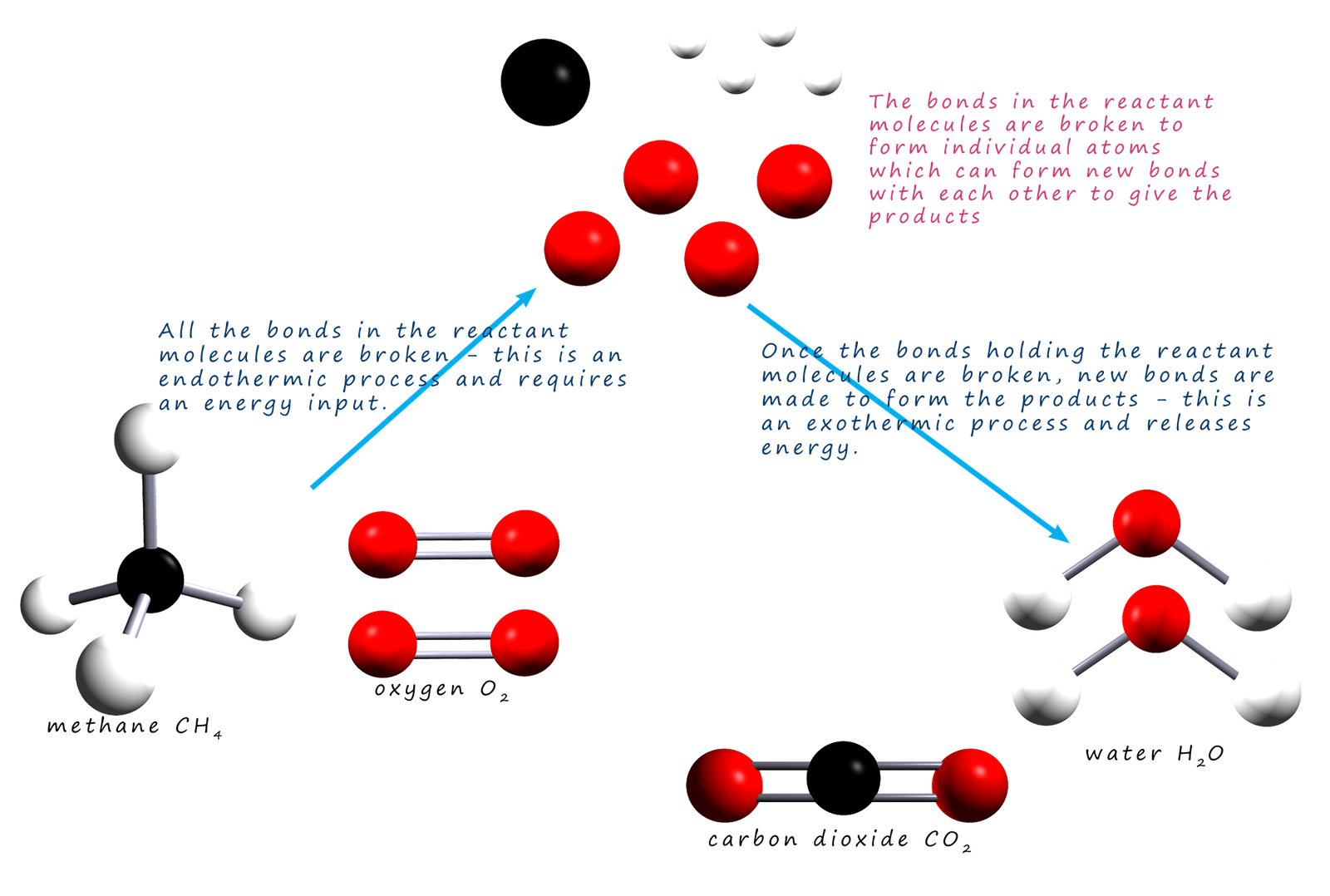
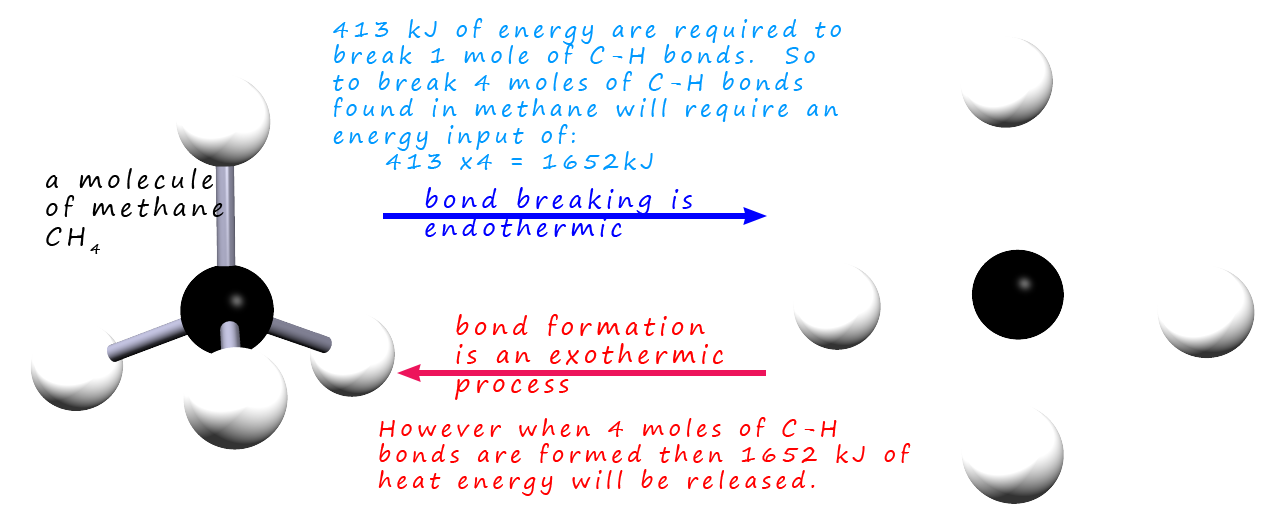
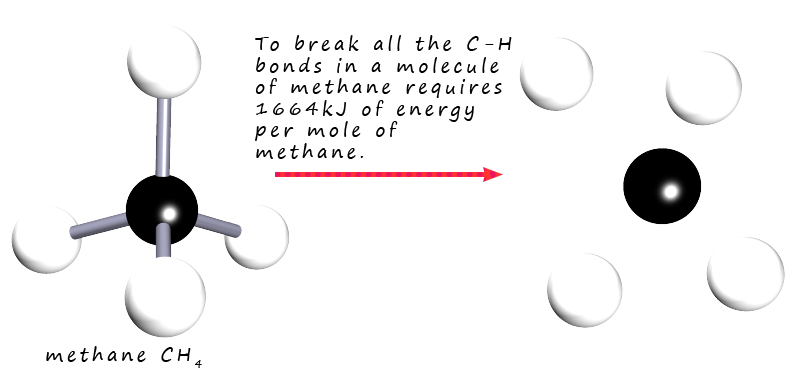
For example, the combustion of methane—methane + 2O₂ → CO₂ + 2H₂O—releases about –890 kJ/mol, a powerful release driving power generation and heating systems worldwide. In contrast, the endothermic dissociation of calcium carbonate, CaCO₃ → CaO + CO₂, absorbing roughly +178 kJ/mol, underpins cement production and high-temperature processes.
These values are not abstract: they shape industrial design and energy calculations.
Measuring Reaction Enthalpies: Methods and Modern Precision
Accurately determining ΔHrxn requires robust experimental and computational tools. Historically, calorimetry reigned supreme—measuring heat flow using controlled isolation of reactants. Bomb calorimeters, for instance, combust samples in sealed chambers at constant volume, capturing all heat released with high precision.
Yet, modern techniques now complement and refine these traditional approaches.
Spectroscopy, particularly infrared and Raman spectroscopy, supplies molecular energy fingerprints by probing vibrational and rotational transitions. These data correlate directly to bond energies, enabling enthalpy estimates without reaction. Computational chemistry thrives on its own strength: quantum mechanical models, such as density functional theory (DFT), simulate molecular geometries and electronic states to compute reaction enthalpies from first principles.
When validated against experiment, such models accelerate discovery in drug development and materials science.
The Hess Law: Building Enthalpies from Intermediate Data
A cornerstone of thermodynamic calculation is Hess’s Law, which asserts that enthalpy changes are path-independent. This principle permits constructing reaction enthalpies from known values of simpler reactions. For example, if ΔH1→2 = –100 kJ/mol and ΔH2→3 = +50 kJ/mol, then ΔH1→3 = –50 kJ/mol, regardless of intermediate steps.
This approach underpins energy balances in complex systems like industrial cycles and biochemical pathways.
Applications Across Industries and Research Frontiers
Reaction enthalpies are invisible architects in energy systems. In fossil fuel combustion, exothermic ΔH values determine heat output and engine efficiency. Power plants optimize fuel mixtures based on enthalpy data to maximize energy recovery and minimize waste.
In chemical manufacturing, ΔH governs reactor design, safety protocols, and cost-efficiency—critical in ammonia synthesis (N₂ + 3H₂ → 2NH₃) where exothermic forward reactions require precise temperature control to prevent runaway reactions.
Biological systems rely equally on enthalpic balance. Cellular respiration, the oxidation of glucose to CO₂ and H₂O, releases –2870 kJ/mol, fueling ATP production. Enzymes fine-tune reaction energetics, lowering activation barriers while preserving thermodynamic favorability—evidence of nature’s intrinsic mastery of energy management.
Environmental and Sustainable Energy Implications
Understanding reaction enthalpies is indispensable for advancing renewable technologies.
Solar fuels, hydrogen production via water splitting (2H₂O → 2H₂ + O₂, ΔH° = +286 kJ/mol), and carbon capture methods all depend on precise enthalpy data to assess feasibility and efficiency. In battery science, Roman battery chemistries and solid-state electrolytes are evaluated through enthalpic analysis to maximize energy density and cycle life.
Emerging green technologies hinge on minimizing energy input while maximizing output—a direct application of enthalpy optimization. Electrochemical CO₂ reduction, for instance, targets favorable ΔH values to convert waste emissions into valuable hydrocarbons, turning environmental challenge into resource opportunity.
The Human and Mechanical Dimension
Behind every reaction enthalpy lies a story of innovation and consequence.
The shift from coal to cleaner fuels, enabled by deep thermodynamic insight, reduces emissions but demands updated engineering. In labs and factories, engineers balance reaction kinetics and thermodynamics to achieve safety, yield, and sustainability. The precision of enthalpy data transforms abstract science into tangible progress—powering homes, healing populations, and sustaining ecosystems.
Reaction enthalpies, encoded in molecular changes, are silent drivers of global industry and natural processes.
By harnessing them through rigorous measurement and computational power, society gains an unparalleled ability to manage energy, respond to environmental imperatives, and push the frontiers of chemistry toward a sustainable future.

Related Post
Argentina Match Live: Watch The Game Here!
Blue_eyed_darkness Age Wiki Net worth Bio Height Boyfriend
Beyond the Beltway: Unpacking Marco Rubio's Children, Their Ages, and the Dynamics of His Family Life

