Unlocking Biological Efficiency: How the Michaelis Constant Reveals Enzyme Power
Unlocking Biological Efficiency: How the Michaelis Constant Reveals Enzyme Power
In the intricate world of cellular metabolism, enzymes act as precise molecular machinists, accelerating biochemical reactions with remarkable speed and specificity. At the heart of understanding their catalytic prowess lies a fundamental concept: the Michaelis constant, or *Kₘ*. This deceptively simple value governs how efficiently an enzyme binds its substrate and transforms it into product, serving as a critical benchmark in biochemistry and enzymology.
From pharmaceutical development to industrial biotechnology, the Michaelis constant is not just a laboratory metric—it is a window into the biochemical soul of enzyme function.
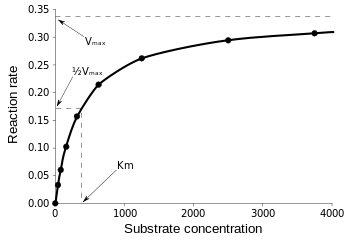
At its core, the Michaelis-Menten equation describes the relationship between substrate concentration and reaction velocity. Expressed mathematically as v = (Vₘₐₓ [S]) / (Kₘ + [S]), *v* represents the reaction velocity, Vₘₐₓ the maximum speed achievable at saturating substrate levels, [S] the substrate concentration, and Kₘ the Michaelis constant.
Often interpreted as the substrate concentration at which the reaction proceeds at half its maximum velocity, Kₘ encapsulates two key kinetic insights: the enzyme’s affinity for its substrate and its intrinsic catalytic efficiency.
Specifically, Kₘ reflects binding strength—lower values indicate tighter substrate binding, while higher values suggest weaker affinity. Yet nuance is essential: Kₘ is not simply a measure of binding, but a composite of association and turnover rates.
To unpack this further, consider the dual processes that define enzyme kinetics: substrate binding and catalytic conversion.
A low Kₘ implies the enzyme reaches half-maximal turnover with minimal substrate—think of a seasoned ballet dancer executing perfect lifts with rare effort. High Kₘ, by contrast, signals reluctance to bind, requiring greater substrate abundance before productivity rises. This dynamic underscores a critical truth: enzymes optimize for environment.
In cellular conditions where substrates may be sparse, enzymes evolve to lower Kₘ values, ensuring metabolic continuity even under scarcity. Conversely, in nutrient-rich environments, Kₘ may rise, reflecting relaxed binding demands. Such adaptability highlights enzyme kinetics as a finely tuned system of biochemical homeostasis.
Deciphering the Values: What Kₘ Really Means in Biological Context
The Michaelis constant is more than a number—it is a story about enzyme-substrate interactions written in molecular terms.Picture two enzymes, identical in function yet differing in Kₘ: one efficiently catalyzes a rate-limiting metabolic step even when substrate is rare, while another performs admirably only when ample substrate floods the system. Biologists use Kₘ values to compare enzyme performance across pathways, organisms, and engineered variants. For example, in glycolysis, hexokinase must efficiently phosphorylate glucose at low concentrations to initiate energy production; its low Kₘ ensures responsiveness.
In contrast, enzymes involved in levels of abundant metabolites might tolerate higher Kₘ, prioritizing speed over sensitivity.
Understanding Kₘ also informs drug design: inhibitors binding tightly to an enzyme often shift the apparent Kₘ, altering reaction kinetics in ways exploitable for therapeutic targeting.
Beyond individual enzymes, Kₘ shapes entire metabolic networks. Cells regulate enzyme expression and post-translational modifications to dynamically adjust Kₘ in response to environmental cues.
This kinetic plasticity enables metabolic flexibility—enabling rapid adaptation to changing nutrient availability, stress, or developmental demands. Researchers leverage Kₘ data to model metabolic flux, optimize bioreactor conditions, and engineer enzymes with tailored substrate preferences for industrial applications, from biofuel production to enzyme-based therapeutics. The constant, though abstract, thus sits at the nexus of molecular function and systems-level physiology.
The Michelis Constant in Real-World Applications
In pharmaceutical research, precise Kₘ measurements guide the optimization of enzyme inhibitors.For instance, statins targeting HMG-CoA reductase rely on detailed kinetic insights to balance potency and specificity, with Kₘ values illuminating how tightly the drug binds under physiological concentrations. Similarly, in synthetic biology, enzyme engineering efforts focus on lowering Kₘ for substrates used in sustainable chemical synthesis, enhancing efficiency and reducing production costs.
Such practical utility underscores a pivotal insight: the Michaelis constant is not merely a research curiosity but a cornerstone of translational science, bridging fundamental biochemistry with tangible innovation.
Despite decades of study, the Michaelis constant remains a lens through which evolving questions in enzymology are addressed.
Advances in rapid kinetics and single-molecule analysis refine our ability to measure and interpret Kₘ under near-physiological conditions, revealing subtleties once hidden by bulk assays. What was once a static parameter now emerges as context-dependent—modulated by pH, temperature, and macromolecular crowding within the cell. This evolving understanding reinforces the necessity of dynamic, systems-oriented approaches in enzyme research.
As both a quantitative marker and a conceptual anchor, Kₘ continues to illuminate how enzymes harness molecular simplicity to sustain the biochemical complexity of life.
In essence, the Michaelis constant is far more than a kinetic equation—it is a testament to nature’s precision in designing reusable molecular machines. By decoding Kₘ, scientists unlock principles that govern how enzymes operate, evolve, and interact within living systems. In this realm, the constant is not a limit but a key, unlocking deeper truths about efficiency, adaptation, and the elegant choreography of biochemistry.



Related Post

Unveiling The Life Of Michael Lavaughn Robinson: The Story Behind The Name
The Ultimate Guide to Navigating the 'Fabulous Forty': 40th Birthday Memes and Hilarious Ways to Celebrate Turning 40

Homebrew CIA QR Code: The Revolutionary Tool Redefining Qualification Access

