Total Dissolved Solids: The Invisible Driver of Water Quality
Total Dissolved Solids: The Invisible Driver of Water Quality
Across ecosystems, industrial channels, and everyday water supplies, a silent yet pivotal factor governs taste, safety, and usability: Total Dissolved Solids (TDS). At its core, TDS refers to the cumulative concentration of inorganic and organic substances dissolved in water—including minerals, salts, and trace chemicals—measured in milligrams per liter (mg/L). Though invisible to the naked eye, TDS profoundly influences water’s sensory and functional properties, shaping everything from brewed coffee bitterness to industrial cooling efficiency.
According to the Total Dissolved Solids Wiki, TDS is not merely a measure of content but a critical indicator of water chemistry and treatment necessity. What Exactly Counts as Total Dissolved Solids? Total Dissolved Solids encompass a broad spectrum of dissolved compounds. The most predominant contributors include cations like calcium (Ca²⁺), magnesium (Mg²⁺), sodium (Na⁺), and potassium (K⁺), and anions such as bicarbonate (HCO₃⁻), sulfate (SO₄²⁻), chloride (Cl⁻), and nitrate (NO₃⁻).
While many of these—especially calcium and magnesium—are naturally occurring in groundwater and surface water, their concentrations determine water quality across regulatory and contextual boundaries. TDS also includes organic fragments from decaying plant matter, microbial byproducts, and anthropogenic pollutants like heavy metals and industrial byproducts. “TDS reflects the full chemical signature of water,” explains Dr.
Elena Ruiz, a hydrogeochemist specializing in aquatic systems. “It’s not just about salt content—it’s the sum of all dissolved ions that affect taste, scale formation, corrosion, and biological compatibility.” This composite nature makes TDS a critical diagnostic tool in water treatment, environmental monitoring, and industrial process control.
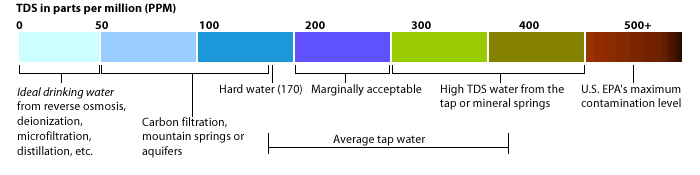
In drinking water, disability to taste typically sets the threshold at 500 mg/L; above this, water is often deemed flat or unpalatable due to mineral dominance. For irrigation, values below 700 mg/L generally support healthy plant growth, while levels exceeding 1,000 mg/L risk salt stress in sensitive crops. Industrial water uses follow distinct benchmarks: cooling towers tolerate TDS up to 1,500–2,000 mg/L, but prolonged exposure can accelerate scale buildup and equipment failure.
Meanwhile, boiler systems demand natively low TDS—often below 250 mg/L—to maintain heat transfer efficiency and prevent premature corrosion. Regulatory agencies such as the Environmental Protection Agency (EPA) and the World Health Organization (WHO) reference TDS data not only for consumer safety but also environmental protection, ensuring water bodies remain ecologically functional. Sources of Total Dissolved Solids: Natural and Human-Made Nature supplies a steady stream of dissolved solids through weathering of rocks, soil leaching, and atmospheric deposition.
Limestone and gypsum beds dissolve over time, releasing calcium and sulfate. Volcanic activity and geothermal springs introduce trace elements like selenium and arsenic. Rainfall further dissolves airborne particulates and airborne salts, contributing to baseline TDS even in remote habitats.
Human activity, however, magnifies TDS concentrations in ways that challenge water sustainability. Urban runoff carries road salts, lawn fertilizers, and road debris into waterways. Industrial effluents from food processing, chemical manufacturing, and power generation discharge concentrated brines and synthetic compounds.
Agricultural drainage introduces nitrates and phosphates, while municipal wastewater—even after treatment—often retains trace organics and dissolved salts. “Human influence has transformed TDS from a natural equilibrium into a calibrated variable,” notes hydrologist Marcus Lin. “Water is no longer just what nature provides, but what society outputs.” Measuring TDS: Techniques and Limitations Accurate assessment of Total Dissolved Solids hinges on standardized laboratory methods.
The most common procedure involves filtration to remove particulates, followed by evaporation of the liquid residue to isolate dissolved solids, weighed and quantified in mg/L. Advanced spectroscopy and ion chromatography now allow real-time, precise ion detection, enabling dynamic monitoring of TDS fluctuations in source and treated waters. Yet measurement carries caveats.
TDS readings reflect an integrated, silicate-like sum—not a per-ion analysis—and do not distinguish between benign and hazardous constituents. For example, sodium-bearing TDS from natural sources poses different risks than those from road de-icers or industrial waste. Additionally, temperature and atmospheric pressure can influence evaporation efficiency, requiring strict calibration to ensure data reliability.
Environmental and health impacts hinge on both TDS concentration and composition. High TDS levels correlate with reduced palatability and increased risk of internal corrosion in pipes, potentially leaching lead or copper into drinking supplies. In sensitive ecosystems, elevated TDS disrupts aquatic flora and fauna—particularly in endorheic basins where mineral accumulation outpaces dilution.
“TDS is a double-edged sword,” cautioned a 2022 report from the Total Dissolved Solids Wiki. “It sustains life in moderation but undermines health and infrastructure when unchecked.” TDS in Daily Life: From Brews to Bathtubs The influence of TDS extends into household routines. Coffee aficionados recognize that mineral-rich water enhances solubility and flavor extraction, with optimal ranges between 75–150 mg/L.
Hard water, high in calcium and magnesium, delivers robust taste but risks limescale buildup in kettles and boilers. Conversely, distilled or demineralized water—effectively TDS-free—imparts flatness but serves critical roles in sensitive electronics and medical apparatus. In personal care, TDS affects skin compatibility: slightly mineralized water supports hydration, while overly saline water can strip natural oils.
Waterfall systems and spa installations rely on controlled TDS to balance aesthetic appeal with physiological safety. Even bottled water marketing hinges on TDS profiles—certified “natural mineral” waters highlight specific cation balances as selling points. The Path Forward: Monitoring and Managing TDS As freshwater scarcity intensifies and urbanization accelerates, Total Dissolved Solids have become a linchpin in water resource management.
Utility providers increasingly deploy real-time TDS sensors to detect contamination spikes and optimize treatment. Industries adopt closed-loop systems to recover valuable minerals and minimize waste discharge. Researchers refine predictive

Related Post
Navigating The PSE Chicago SE Finance Department: A Comprehensive Guide
Spartanburg Mugshot: Unveiling the Face Behind the Badge in South Carolina’s Justice System
Yellowstone Season 1's Brutal Ending: A Ranch In Crisis and a Family Redefined

