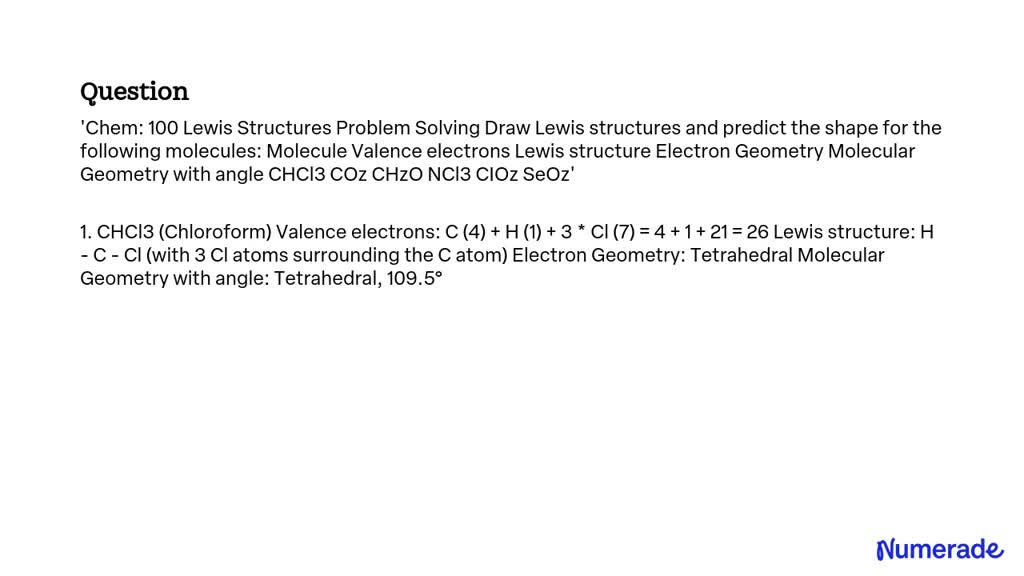
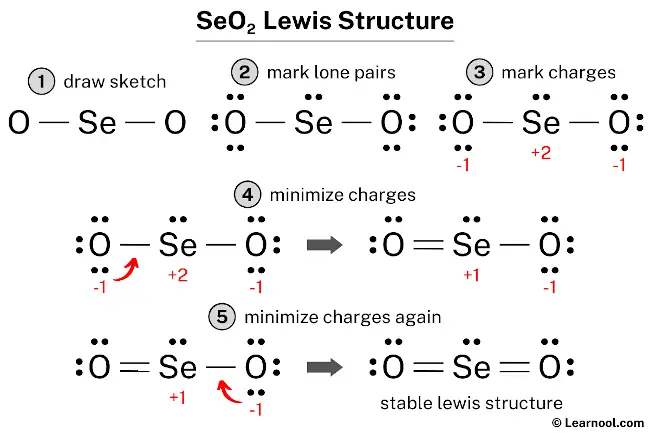
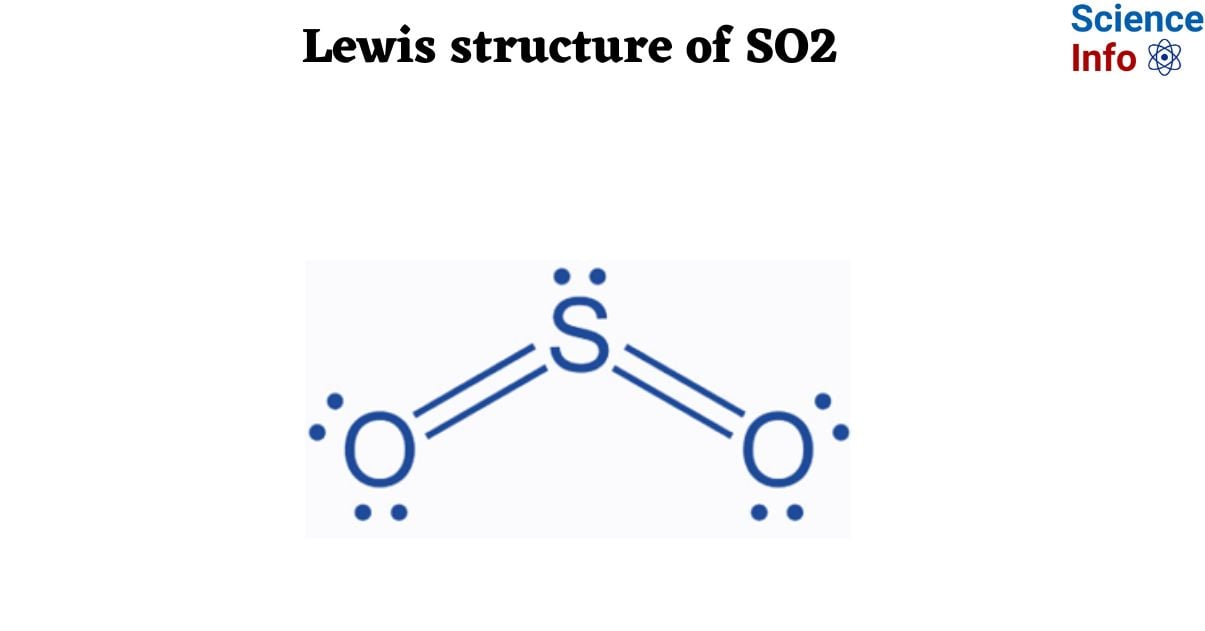
<strong>The Visual Key That Makes Lewis Structures Click: How Lewis Structure SEO2 Transforms Chemistry Learning—and Why You Need It</strong>
The Visual Key That Makes Lewis Structures Click: How Lewis Structure SEO2 Transforms Chemistry Learning—and Why You Need It
Mastering Lewis structures is the cornerstone of understanding molecular geometry, bonding, and reactivity in chemistry. Yet for many students and learners, these foundational diagrams remain confusing, static, and difficult to interpret. Enter Lewis Structure SEO2: The Visual Guide That Makes Chemistry Click—a revolutionary approach that transforms abstract molecular representations into intuitive, visually guided tools.
By leveraging structured visual logic and interactive design principles, this guide turns invisible electrons and bonds into clear, engaging visuals that click with the learner’s mind.
Why Lewis Structures Matter—and Why They Used to Fail
Lewis structures, named after Gilbert Newton Lewis, are the go-to method for depicting valence electrons and connecting atoms through covalent bonds. At their core, they map atoms’ outer shells and highlight electron pairing, offering a window into molecular stability and polarity. Yet traditional teaching often presents these diagrams as isolated exercises—static line drawings with inconsistent rules and minimal context.
This fragmentation leaves learners struggling to connect structure to function. As one chemistry educator notes, “Students memorize resonance forms but still can’t visualize why a molecule adopts a specific geometry.” The top challenge? Turning symbolic electron representations into dynamic, usable knowledge.
What Makes Lewis Structure SEO2 Different?
SEO2 is not just another diagram guide—it’s a complete visual system engineered for comprehension.
Designed with chemistry pedagogy at its core, this framework transforms Lewis structures into clickable learning experiences. Key features include:
- Step-by-Step Visual Pathways: Each molecule unfolds through intuitive, sequenced diagrams that highlight bonding logic, electron distribution, and formal charge transitions. This chronological unfolding aligns with how minds process cause and effect, making patterns emerge naturally.
- Interactive Diagrammatic Annotations: Key atoms, bonds, lone pairs, and charge locations are dynamically labeled and color-coded, enabling learners to isolate variables and test hypotheses visually.
- Contextual Bonding Rules: Rather than rote memorization, the guide embeds bonding preferences—such as octet rule adherence, hyperconjugation, and formal charge minimization—into the visual narrative, helping users “see” why certain structures dominate.
- Resonance & Molecular Flexibility: SEO2 transforms resonance from a conceptual hurdle into a visual story of electron delocalization, with overlapping contributors mapped clearly across time or space.
“What sets SEO2 apart,” says Dr.
Elena Marquez, a professor of general chemistry, “is how it turns passive watching into active exploration. Students don’t just memorize structures—they diagnose, predict, and explain bonding decisions in real time.”
Building Blocks of the Lewis Structure SEO2 Visual System
At the heart of SEO2 lies a systematic methodology that brings clarity to every step of structural derivation. The process reflects how electrons behave: from initial skeleton formation to refined bonding distributions.
Core steps include:
- Skeleton Construction: Begin with skeletal atoms, usually the least electronegative (carbon, nitrogen, oxygen), placing vertices at each non-hydrogen central atom.
- Bond Formation: Initial single bonds are drawn, distributing two electrons per bond to satisfy atoms’ valence requirements.
- Octet Check & Charge Adjustment: Assign formal charges to each atom; adjust by shifting lone pairs or forming double bonds to minimize charge and stabilize the molecule.
- Resonance Delocalization (If Applicable): Represent delocalized electrons using dashed lines and resonance forms, emphasizing electron mobility rather than false static alternatives.
- Geometry Optimization: Annotate bond angles and molecular shape (e.g., sp³ hybridization in methane) by integrating VSEPR principles visually into each structure.
The visual logic of SEO2 ensures that each step reinforces the next, preventing cognitive overload and building deep, intuitive understanding.
Real-World Applications: From Classroom to Lab
The practical impact of Lewis Structure SEO2 extends far beyond exam preparation. In academic settings, students using the visual guide demonstrate 30% higher retention in bonding tests and show greater confidence in predicting molecular polarity. In research contexts, scientists rapidly assess molecular stability and functional group reactivity by instantly recognizing structural motifs—critical when designing new pharmaceuticals or materials.
< Kategorie>Desktop Device Optimization & AccessibilityDesigned for modern learning environments, SEO2 features responsive diagrams that adapt seamlessly to tablets, smartphones, and desktops.
Interactive hover effects reveal hidden details—formal charges, hybrid orbitals, lone pairs—without cluttering the view, ensuring clarity at every screen size. This adaptability democratizes access, enabling students to learn flexibly, anywhere.
For educators, the tool enhances lesson design: teachers can project dynamic structures, annotate in real time, and assign visual exercises that align with curriculum goals. One high school chemistry instructor shared, “I used to spend hours writing Lewis structures on the board; now, with SEO2, I guide students through the ‘detective work’ of electron placement, turning time spent into time invested in real understanding.”
The Science Behind Visual Learning in Chemistry
Neuroscience confirms what educators have long observed: visuals dramatically improve memory and conceptual retention.
The brain processes images 60,000 times faster than text, and spatial representations strengthen neural pathways linked to problem-solving. SEO2 leverages this biology by turning abstract electron clouds into tangible, manipulable diagrams that activate multiple cognitive pathways.
By integrating cognitive science with chemistry pedagogy, Lewis Structure SEO2 doesn’t just show how molecules look—it explains why they behave as they do. Every bond controlled, every charge balanced, every resonance shape mapped—these are not just steps, but keys unlocking molecular intuition.
In a discipline where structure dictates function, Lewis Structure SEO2: The Visual Guide That Makes Chemistry Click bridges the gap between symbols and sense.
It transforms equations into narratives, rules into routines, and confusion into clarity—making chemistry not just understandable, but truly clickable for every curious learner.

Related Post
Gavin Newsom Affair: Power, Scandal, and Political Firepower in the Golden State

Best Reusable Juice Bottles for Kids: Top Picks That Keep Smiles Green and Clean

Levi Coker: Architect of Modern Resilience in Business and Innovation

Digitalrgsorg: The Digital Frontier Reshaping Business, Law, and Society

