The Universal Language of Chemistry: Mastering Unit Conversions with the Essential Chemistry Unit Conversion Chart
The Universal Language of Chemistry: Mastering Unit Conversions with the Essential Chemistry Unit Conversion Chart
Understanding the precise relationships between chemical quantities is not just a technical skill—it’s the cornerstone of accurate scientific practice, industrial efficiency, and educational mastery. At the heart of this fluency lies the Chemistry Unit Conversion Chart, a vital reference that transforms abstract measurements into actionable, standardized values. From grams to moles, liters to nanoliters, this tool turns variables into quantifiable truths, enabling chemists, researchers, students, and practitioners across industries to communicate results with clarity and confidence.
Whether calibrating reagents in a laboratory or scaling formulas for large-scale manufacturing, mastery of unit conversion ensures reliability, consistency, and safety.
The necessity of standardized unit conversion in chemistry cannot be overstated. A single misassumed conversion—such as confusing milliliters with liters or moles with grams—can derail experiments, compromise product quality, or even pose hazards in research and production environments.
As the American Chemical Society (ACS) emphasizes, “Accuracy in measurement underpins every scientific discovery and industrial process.” The Chemistry Unit Conversion Chart serves as a permanent, accessible anchor in this pursuit, offering a visual and numerical framework that bridges disparate systems: the metric, imperial, and even rare laboratory units. It transforms units from barriers into bridges, ensuring seamless translation between measurement worlds.
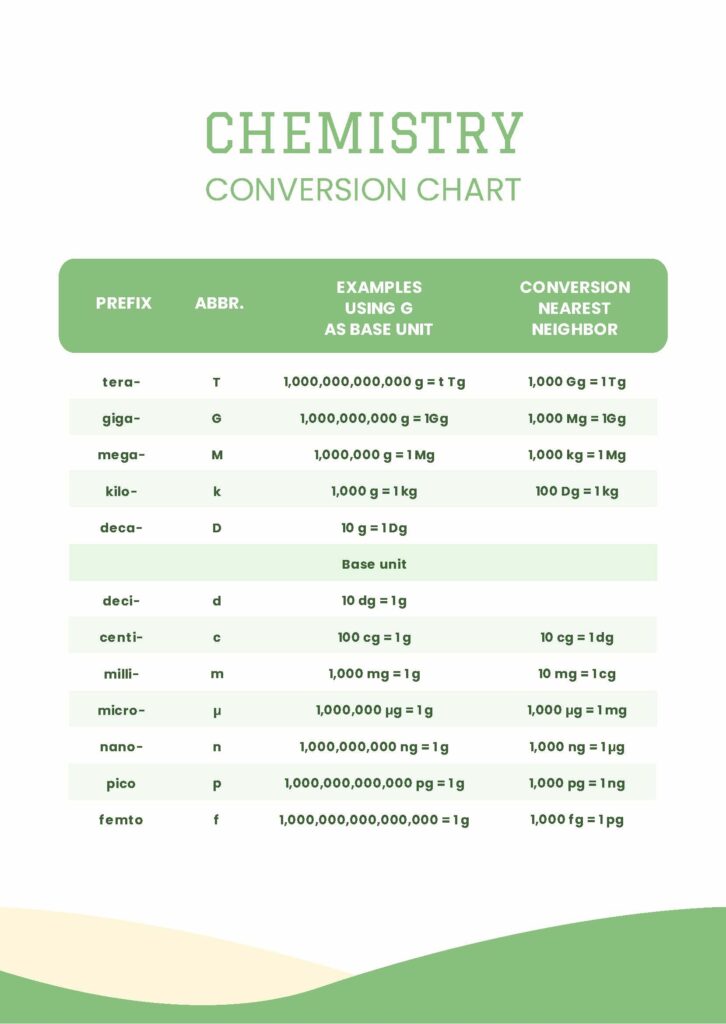
Decoding the Structure of a Modern Chemistry Unit Conversion Chart
A properly designed Chemistry Unit Conversion Chart organizes core physical quantities in a coherent, navigable layout.Typically structured in a grid format, it maps units across dimensions such as mass, volume, moles, area, concentration, and temperature. Each row represents a base or derived quantity, while columns allow transformation across standardized systems—most commonly the metric system and, where applicable, imperial units like milliliters, pints, or gallons. For example, the conversion between grams and moles relies on the molar mass, a fundamental bridge between mass and number of particles.
The chart explicitly notes that one mole of any substance contains approximately $6.022 \times 10^{23}$ entities (Avogadro’s number), enabling direct switching between grams and moles via mass-to-mole calculations. This is not merely algebraic manipulation; it reflects a deep understanding of stoichiometry and atomic scale relationships. Component categories commonly included in such charts are: - Mass: grams (g), milligrams (mg), kilograms (kg), tons - Volume: milliliters (mL), liters (L), cubic centimeters (cm³), cubic meters (m³) - Moles: identified by molar mass and molecular weight - Concentration: molarity (mol/L), molality (mol/kg solvent), grams per liter (g/L) - Temperature: Celsius (°C), Kelvin (K), Fahrenheit (°F), with conversion factors embedded - Time: seconds, minutes, hours—critical for reaction kinetics In practice, the chart enables rapid, error-resistant conversions by indicating primary conversion factors at a glance.
For instance, the text or symbol between “gram” and “mole” might appear as: One mole of water (H₂O) = 18.015 grams ≈ 18 g/mol. This precise linkage eliminates guesswork, particularly in high-stakes settings like pharmaceutical development or chemical synthesis.
Consider a scenario where a lab technician prepares a 0.5 M solution of sodium chloride (NaCl), a compound ubiquitous in both lab and industry.
Without proper unit conversion, the technician might struggle to determine the correct mass needed for a given volume. However, consulting a Chemistry Unit Conversion Chart reveals that: - Molarity = moles per liter - Molar mass of NaCl ≈ 58.44 g/mol - Thus, 0.5 mol/L × 58.44 g/mol = 29.22 grams per liter
This integrated approach cuts time, reduces human error, and reinforces procedural accuracy.From Theory to Application: Real-World Benefits Across Scientific Disciplines
The practical impact of the Chemistry Unit Conversion Chart spans multiple domains, each demanding precision and consistency.In education, students transitioning from basic stoichiometry to complex laboratory work depend on these charts to internalize unit relationships. Conversion fluency transforms abstract mole ratios into tangible measurements—e.g., when balancing chemical equations, students convert grams of reactants to moles to liters of gas, solidifying conceptual understanding through computational practice. In research laboratories, conformity to standardized conversions ensures reproducibility across teams and institutions.
When collaborating internationally, researchers from countries using different systems—such as U.S. conversion (mL, °F) versus metric (mL, °C)—can align measurements instantly through a shared visual tool. This eliminates ambiguity and supports global scientific cooperation.
Industrial applications further underscore the chart’s indispensability. Pharmaceutical manufacturers, chemical engineers, and environmental scientists rely on standardized conversions to scale formulations, optimize reactions, and comply with regulatory standards. For example, converting nanograms to grams is essential in drug dosage development, where trace accuracy directly affects efficacy and safety.
Similarly, in environmental chemistry, formulas involving parts per million (ppm) or milligrams per liter (mg/L) demand exact unit alignment to monitor pollutants and safeguard public health. ^“A chemistry lab without a conversion chart is like a navigator without a compass—error-prone and inefficient,”* notes Dr. Elena Ramirez, a chemical education specialist.
Her insight underscores the chart’s role not just as a reference, but as a foundational instrument in scientific literacy.
Key Conversion Factors and Techniques in Everyday Practice
Effective use of the Chemistry Unit Conversion Chart hinges on familiarity with core conversion factors and the ability to chain units strategically. Key transformations include: - Volume to mass and vice versa: understanding $1 \text{ L} \approx 1 \text{ kg}$ for water (density ~1 g/mL), and mass-to-volume relations via density.- Molar mass calculations: converting grams to moles and vice versa using atomic masses from the periodic table—e.g., carbon (12 g/mol), oxygen (16 g/mol) in CO₂. - Concentration conversions: converting between mol/L and g/L using molar mass, or between mg/L and ppm (where 1 mg/L = 1 ppm). - Temperature scales: maintaining precision when converting between °C and K (°K = °C + 273.15), critical for reaction rate studies dependent on thermal conditions.
- Time and rate conversions: linking minutes to hours or seconds to minutes in kinetics and reaction yield calculations. Practical strategies include: - Memorizing common molar masses (e.g., H₂O: 18 g/mol, NaCl: 58.44 g/mol) - Using dimensional analysis—writing units as a fraction to cancel irrelevant terms—ensuring dimensional consistency - Maintaining a version of the chart visible during calculations to reduce cognitive load and error risk Mastery of these techniques transforms unit conversion from a rote exercise into an intuitive, reliable science.
In the precision-driven world of chemistry, the Chemistry Unit Conversion Chart transcends mere utility—it becomes a standard of practice, ensuring that what begins as disparate units converges into shared, unambiguous truth.
From classroom exercises to industrial megaproduction lines, this tool sustains accuracy, accelerates workflows, and strengthens global scientific collaboration. As measurements evolve and new compounds emerge, the chart endures as a constant, empowering professionals to translate variability into clarity, one perfectly conversion at a time. The chemistry of understanding lies not just in elements and bonds—but in the bridges built across units and minds.



Related Post

