The Essential Baking Soda and Vinegar Experiment: A Chemical Carnage of Color and Foam
The Essential Baking Soda and Vinegar Experiment: A Chemical Carnage of Color and Foam
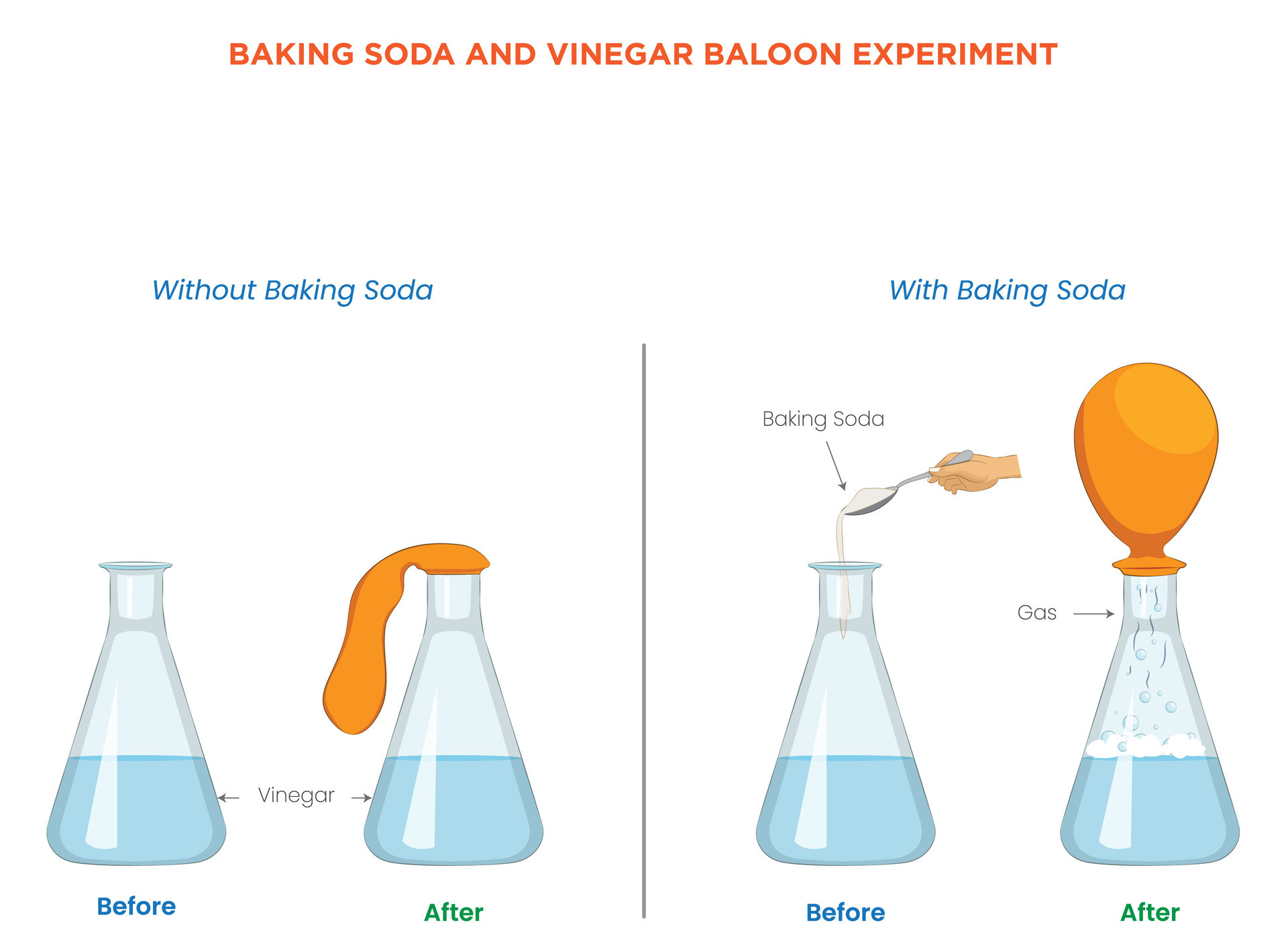
When baking soda meets vinegar, one word captures the explosive surprise: fizz. More than just a kitchen mishap, the classic baking soda and vinegar reaction is a dynamic science experiment that vividly demonstrates acid-base chemistry in action. This simple, accessible demonstration—accessible in any home lab—uncovers the dramatic transformations when a weak base and a weak acid combine, producing carbon dioxide gas, temperature shifts, and a rush of vibrant bubbling.
Far from just a fun classroom trick or kitchen experiment, this reaction serves as a foundational window into chemical processes that affect everyday life—from fermentation to carbonated drinks. Understanding the science behind this explosive interaction enriches appreciation for the invisible forces shaping the world around us. The core of the experiment lies in a proficient acid-base reaction: baking soda, chemically known as sodium bicarbonate (NaHCO₃), acts as a base, while vinegar—acetic acid dissolved in water—serves as the acidic counterpart.
When mixed, they undergo a rapid chemical transformation that generates carbon dioxide gas (CO₂), water (H₂O), and sodium acetate as a byproduct. This second product contributes to the sluggish effervescence long associated with the experiment, though minimal than in stronger acid-base combinations.
Chemical Mechanics: What Happens at the Molecular Level
The molecular dance begins with dissociation.
Sodium bicarbonate dissociates in solution into sodium ions (Na⁺) and bicarbonate ions (HCO₃⁻). When vinegar’s acetic acid (CH₃COOH) enters the mix, it partially ionizes, releasing hydrogen ions (H⁺). These H⁺ ions combine with bicarbonate to form carbonic acid (H₂CO₃), an unstable compound that immediately decomposes into water and carbon dioxide gas:
- CH₃COOH + HCO₃⁻ → H₂CO₃ → H₂O + CO₂↑
- CO₂: The rapid escape of carbon dioxide gas creates the trademark fizz, visible bubbles, and the pressure-driven foam formation.
As noted by physical chemist Dr.
Elena Torres, “The effervescence is not just a visual flourish—it’s a measurable indicator of hydrogen ion consumption and carbonate species depletion, offering a tangible demonstration of neutralization kinetics.”
Temperature plays a subtle but significant role. This reaction is exothermic, meaning it releases heat. But unlike more intense combustion or neutralization reactions, the heat output is moderate—typically raising solution temperatures by just 3°C to 5°C under standard conditions.
This controlled release of thermal energy reinforces the idea that not all acid-base interactions produce extreme heat; some are surprisingly mild but visually dramatic.
Multi-Modal Exploration: Variables That Shape the Reaction
The reaction’s intensity and behavior depend on precise experimental conditions. Key variables include:
- Concentration: Using highly concentrated acetic acid intensifies bubble production, while



Related Post
Skyward Mcallen: Pioneering Excellence in Aviation and Beyond
May 12 Zodiac Insight: Unlocking Taurus Relationships Through Astrological Compatibility
A Timeless Talent in Hollywood: The Enduring Legacy of Joyce Van Patten
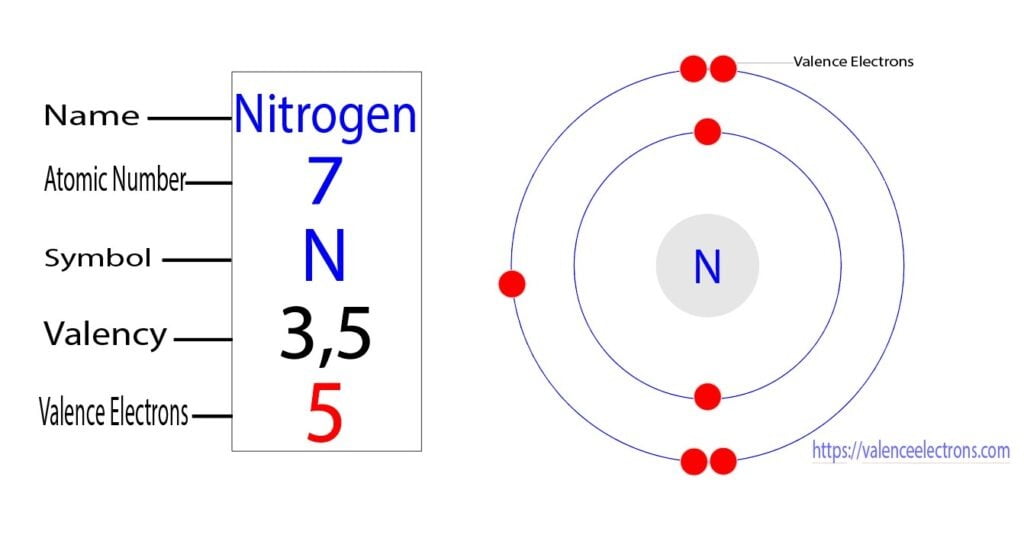
S Valence Electrons: The Hidden Forces Shaping Chemical Identity

