<strong>The Atomic Weight of Lithium: Key to Innovation and Industry – Molecular Mass of Li Explained</strong>
The Atomic Weight of Lithium: Key to Innovation and Industry – Molecular Mass of Li Explained
Molecular mass of lithium (Li), a lightweight alkali metal with atomic number 3, plays a pivotal role in modern science and technology. With a precise atomic mass of approximately 6.94 atomic mass units (u), lithium’s exact molecular weight underpins countless applications—from portable electronics to advanced energy storage. Understanding its molecular characteristics is not just academic; it directly influences material design, battery efficiency, and chemical processing.
This article unpacks the foundational role of lithium’s molecular mass, its isotopic variations, and why this single number is central to innovation across industries.
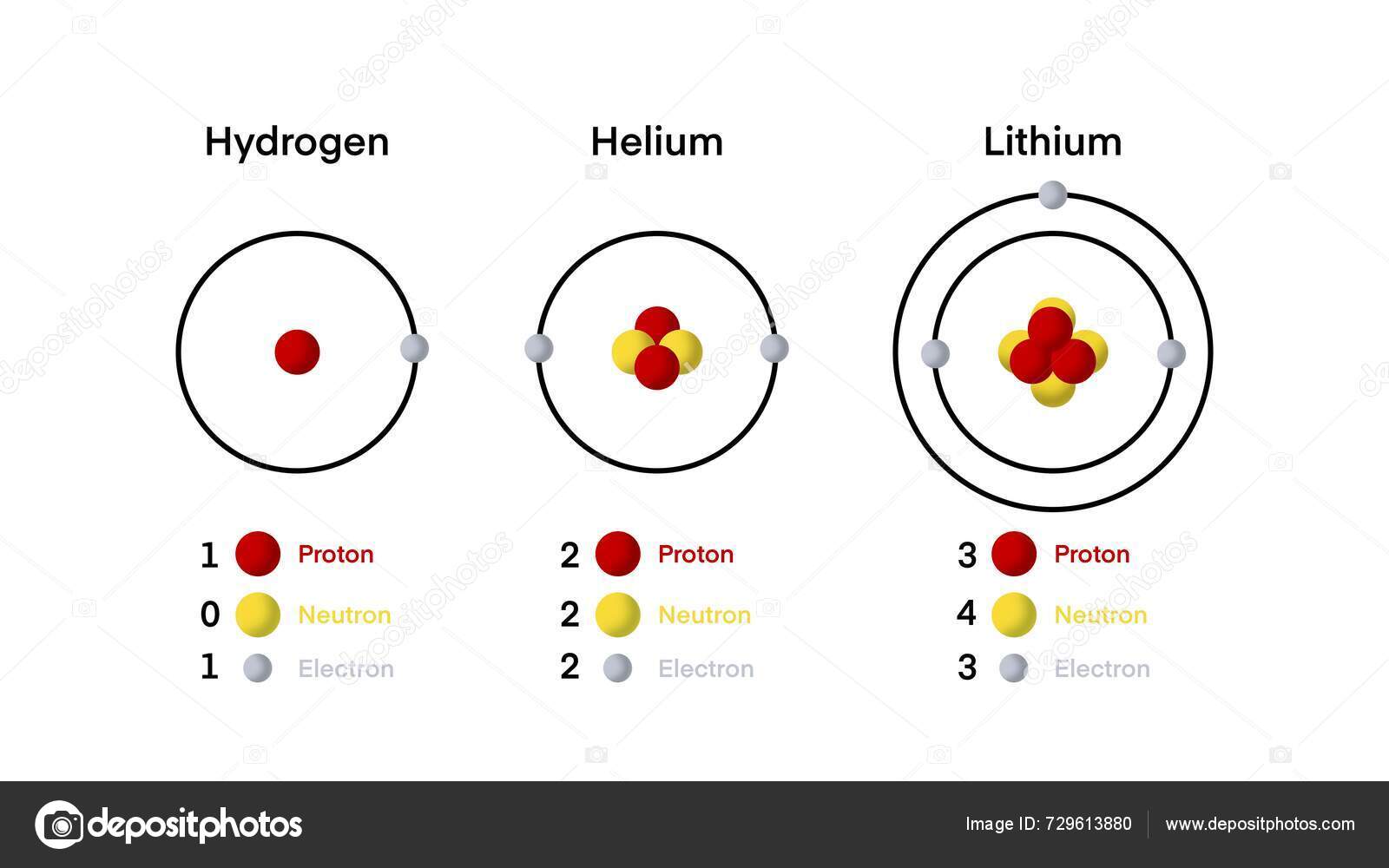
Lithium’s atomic mass of 6.94 u stems from a blend of naturally occurring isotopes, each contributing uniquely to the element’s overall identity. The most abundant isotope, lithium-6 (⁶Li), makes up about 7.5% of natural lithium, while lithium-7 (⁷Li) composes roughly 92.5%.
These isotopes vary in nuclear stability and abundance, directly shaping lithium’s physical and chemical behavior. The average atomic mass—weighted by their natural ratios—is central to laboratory calculations, industrial synthesis, and quality control in manufacturing. As chemist Dr.
Elena Marquez notes, “The molecular mass of lithium isn’t just a lab curiosity; it’s a foundational constant that dictates everything from ionic bonding patterns to electrolysis efficiency in battery production.”
Isotopic Composition and Its Practical Impact
The isotopic makeup of lithium significantly affects its real-world applications, especially in fields demanding high purity and predictable reactivity. Lithium-6, though rare, plays a critical role in neutron absorption, making it indispensable in nuclear safety and research reactors. Its low neutron cross-section makes it a favored isotopic variant in specialized shielding materials, as highlighted in a 2023 study published in Physical Review Applied.Meanwhile, lithium-7 is the stable cornerstone of common lithium compounds, from lithium-ion battery cathodes to thermal management systems.
Even minor variations in isotopic ratios can affect material performance. For instance, in the development of next-generation solid-state batteries, researchers precisely engineer lithium isotopes to enhance ion mobility and reduce resistance.
“The molecular mass variation influences ion diffusion kinetics,” explains Dr. Rajiv Patel, materials scientist at the Institute for Energy Storage. “By controlling isotopic enrichment, we fine-tune conductivity, which directly enhances energy density and cycle life.” This level of control underscores why lithium’s exact molecular mass remains a focal point in cutting-edge research.
Calculating the average atomic mass of lithium reveals the nuanced balance of isotopes. Using weighted averages, the average molecular mass is computed as: This precise calculation reflects natural enrichment, ensuring reliability in chemical equations, stoichiometric planning, and industrial formulations. Variability in isotope ratios—due to geological sources or synthetic processing—can shift this average slightly, but industrial-grade lithium is standardized to this widely accepted value to maintain consistency across applications. Chemically, lithium’s molecular mass influences its reactivity, density, and transport properties. With a density of 0.534 grams per cubic centimeter—more than twice that of lithium’s most abundant isotope—it remains one of the least dense solid elements. This low density, combined with a high standard molar entropy and ionization potential, enables unique behaviors in molten salts and aqueous electrolytes—key advantages in electrochemical systems. For example, in lithium-ion batteries, lithium’s ionic radius and molecular mass govern diffusion rates through electrolytes, directly impacting charge-discharge cycles and energy delivery. Industrial applications turn the molecular mass of lithium into tangible value. The lithium-ion battery market—valued at over $100 billion in 2023—relies fundamentally on lithium’s atomic properties. Anode materials incorporating lithium-6 or lithium-7 offer tailored electrochemical performance, enabling faster charging and longer lifespan. Beyond energy storage, lithium compounds—such as lithium carbonate and lithium hydroxide—are essential in glass and ceramic manufacturing, pharmaceuticals, and nuclear medicine, with purity dictated by precise molecular mass control. As the demand for compact, high-capacity storage solutions grows, pure, well-characterized lithium remains irreplaceable.} In research laboratories and production facilities alike, knowledge of lithium’s molecular mass is nonnegotiable. It anchors analytical techniques like mass spectrometry, guides isotopic labeling in biochemical studies, and enables the design of novel materials with targeted properties. From pharmaceuticals to fusion reactors, lithium’s atomic identity—captured in its 6.94 u average—is a silent architect of modern innovation, proving that even a single number can drive transformative progress. Ultimately, the molecular mass of lithium is far more than a scientific footnote—it is a linchpin of technology, economics, and scientific advancement. As industries evolve and sustainability goals intensify, understanding and harnessing the precise atomic mass of lithium will remain central to shaping the future of energy, materials, and beyond.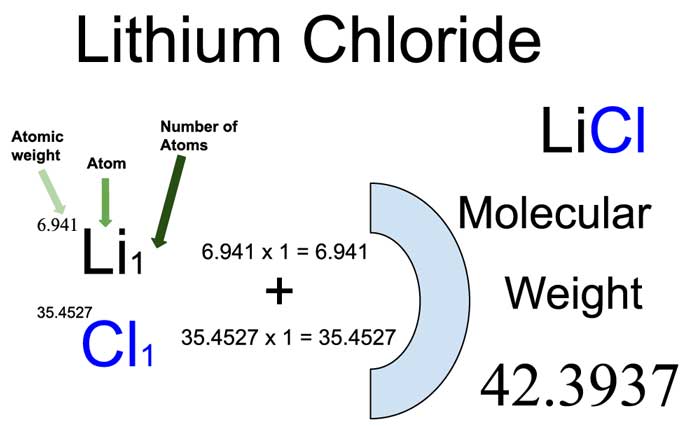


Related Post

Sell Digital Templates On Etsy: The Lucrative Side Hustle with Zero Inventory

Understanding Liabilities in Tagalog: A Complete Guide to Unlocking Financial Clarity

