So2 Lewis Structure: Decoding the Molecular Blueprint of Sulfur Dioxide
So2 Lewis Structure: Decoding the Molecular Blueprint of Sulfur Dioxide
Sulfur dioxide, a simple yet fascinating molecule with the formula SO₂, plays a pivotal role in atmospheric chemistry, industrial processes, and environmental science. At first glance, its structure appears deceptively simple—two oxygen atoms bonded to a central sulfur atom—yet its behavior and interactions reveal complex electron behaviors best illuminated by So₂’s Lewis structure. Understanding this structure is essential not only for chemists but also for educators, students, and professionals tracking air quality and chemical reactivity.
This article delves into the precise atomic arrangement of SO₂ through Lewis theory, explains how bond formation governs its reactivity, and explores the broader implications of its molecular blueprint.
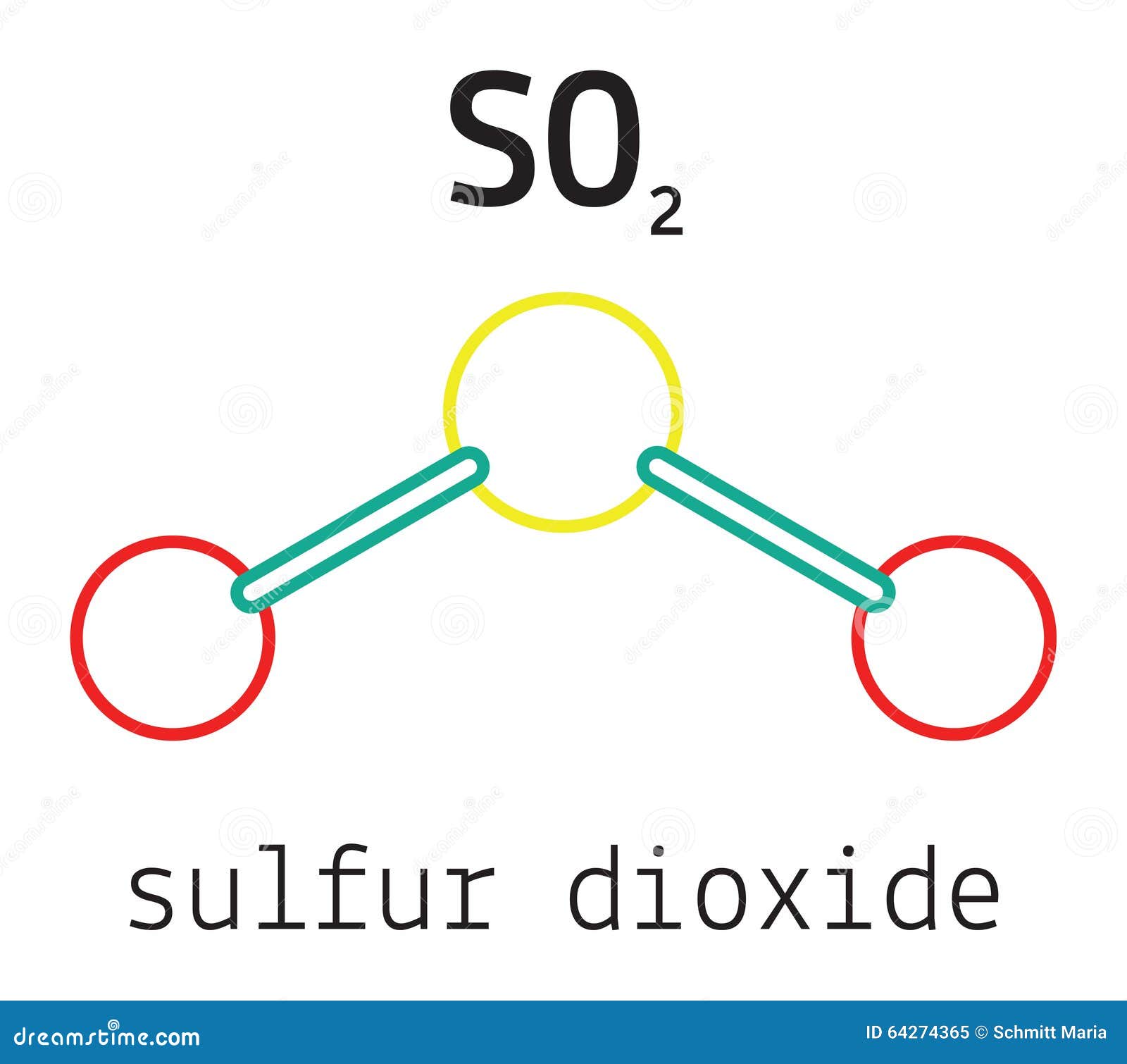
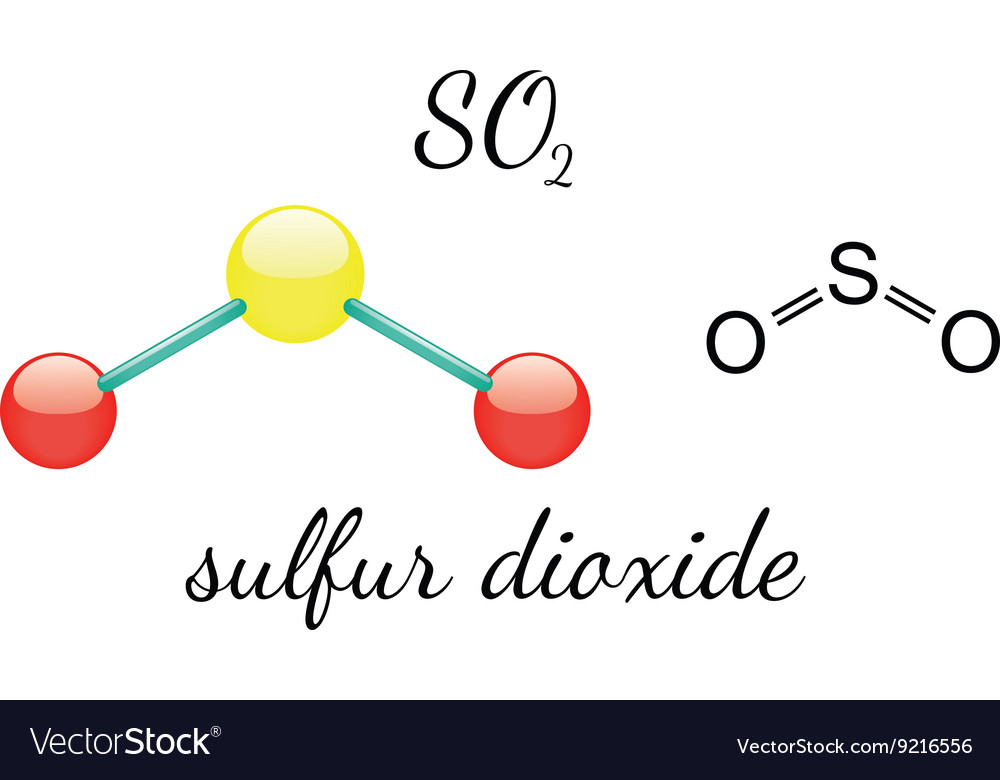
The Lewis structure of SO₂ provides a foundational model for predicting how the molecule shapes its chemical interactions. Centered on sulfur, the backbone of the structure, lies two oxygen atoms—each sharing a pair of electrons with sulfur in a bonding configuration, while one oxygen carries a lone pair of electrons.
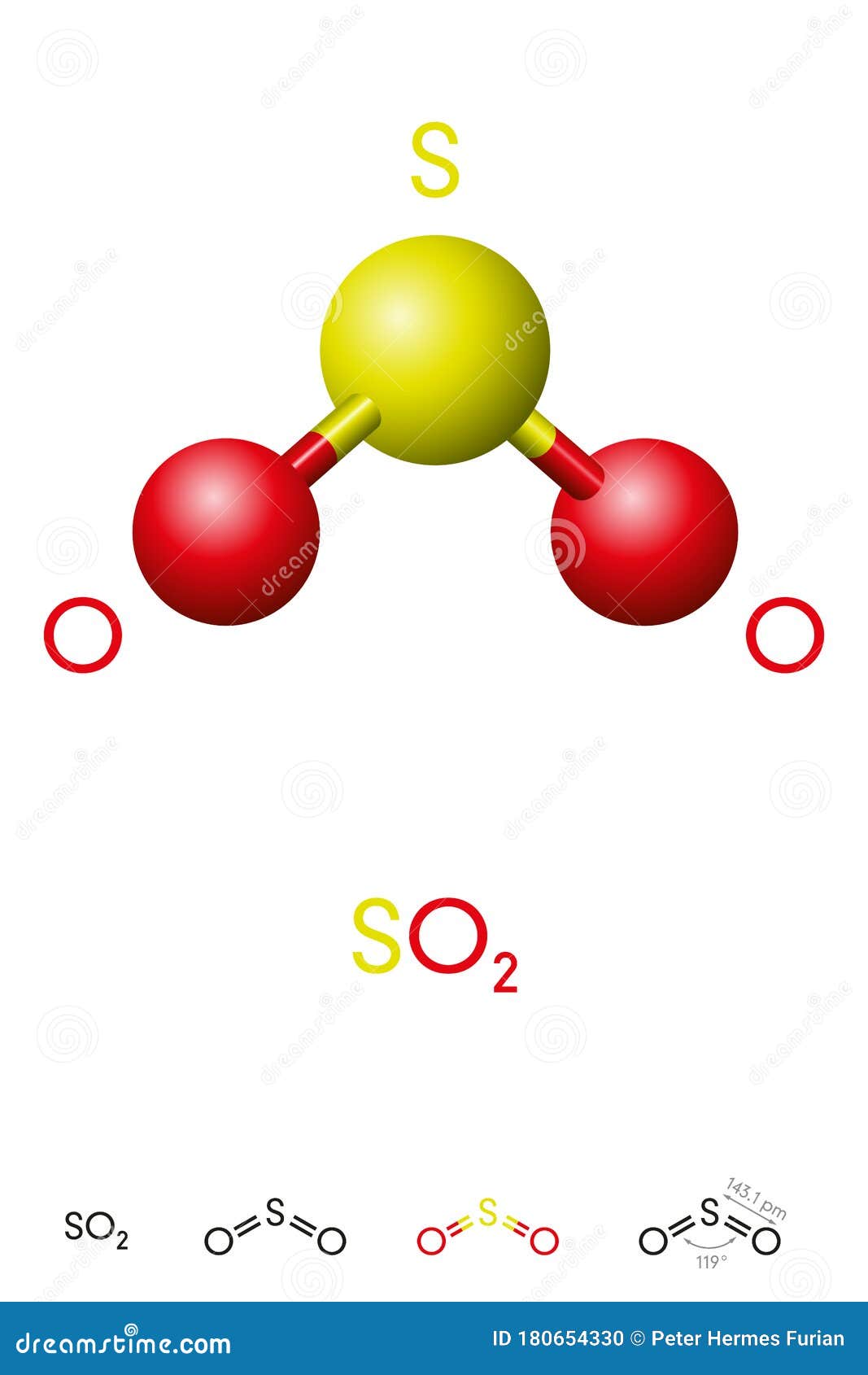
The result is a bent molecular geometry, deviating from the idealized trigonal planar arrangement due to the presence of a lone electron pair on sulfur. “In SO₂, the lone pair on sulfur increases electron repulsion, compressing the bond angle to approximately 119 degrees,” explains physical chemist Dr. Elena Marquez, “making it a classic example of how valence shell electron pair repulsion (VSEPR) theory predicts molecular shape.” Engaging the core of the Lewis model, sulfur in SO₂ exists in a +4 oxidation state, accepting electrons from each oxygen.
Oxygen, fulfilling the standard octet rule (with a couple of exceptions in expanded octets seldom seen here), contributes two valence electrons per atom. Sulfur, with six valence electrons, shares two through each of two single bonds—totaling four shared electrons—and retains two lone electrons, completing its octet. This electron count follows the fundamental principle: atoms bond to achieve stable electron configurations, typically mirroring or surpassing the pairing found in noble gas configurations.
Delving deeper, the two O–S bonds in SO₂ are not merely single covalent links—they carry partial double-bond character due to resonance. Resonance stabilizes the molecule by delocalizing electrons across both sulfur-oxygen connections. Though formally depicted with one double bond, spectroscopic data confirm a bond order averaging 1.5, indicative of electron sharing distributed across both bonds.
“This resonance lowers the overall energy of the molecule and enhances its stability,” notes Dr. Rajiv Patel, a computational chemist specializing in reactive intermediates. “It explains why SO₂ doesn’t rapidly decompose under standard conditions, yet remains biologically active.”
Resonance in SO₂ has more than theoretical weight—it directly influences its reactivity.
The molecule’s bent form and lone pair on sulfur create a region of heightened electron density, making SO₂ a good molecular electron donor in certain chemical environments. It readily reacts with water vapor to form sulfurous acid (H₂SO₃), a transformation critical in atmospheric processes. “SO₂’s trigonal bent geometry positions the oxygen with a negative formal charge, synergistically attracting protons from moisture,” observes environmental chemist Dr.
Lisa Chen. “This protonation pathway is central to its role in acid rain formation.”
Structurally, the geometry and electron distribution in SO₂ further dictate its physical properties. With a bond angle of ~119 degrees, the molecule exhibits polar character—a permanent dipole moment arises from oxygen’s electronegativity and the bent shape.
This polarity makes SO₂ moderately soluble in water, essential for its transport in the atmosphere. In solid or liquid phase, SO₂ crystallizes into bent dimers or interacts through weak dipole-dipole forces, minimizing lattice strain.
The importance of SO₂’s Lewis structure extends beyond the lab.
In pollution monitoring, accurate structural models allow precise predictive modeling of emission dispersion and atmospheric transformation. In combustion and energy sectors, understanding electron flow through SO₂ bonds informs strategies to reduce sulfur emissions—a key factor in limiting mercury vapor release and sulfate aerosol production. “Accurate molecular models like the Lewis structure of SO₂ are not just academic tools—they’re operational assets,” asserts Dr.
Marquez. “They bridge molecular insight with real-world mitigation.”
Modern spectroscopic techniques, including infrared and microwave spectroscopy, have validated the Lewis structure’s predictions. Experimental data consistently show bond angles near 120 degrees, minor bond length variations consistent with resonance averaging, and lone pair behavior aligning with tensored electron density maps.
“The agreement between theory and observation strengthens confidence in using SO₂’s Lewis structure as a reliable guide,” adds Dr. Chen.
In education, the SO₂ Lewis structure serves as a gateway to more advanced topics.
It illustrates key principles—VSEPR theory, symbolism, electron counting—and scaffolds learning toward molecular orbital theory and reactivity patterns. For students, visualizing sulfur’s expanded octet flexibility and oxygen’s electron dynamics demystifies abstract bonding concepts. “It’s a perfect teaching example,” says Dr.
Patel. “From geometry to reactivity, SO₂’s layered structure teaches how atoms cooperate through electron sharing—and how that cooperation shapes environmental and industrial outcomes.” In essence, the So₂ Lewis structure is far more than a diagram; it is a dynamic, evidence-based representation of how electrons govern molecular behavior. By decoding this molecular blueprint, scientists and students gain a precise lens through which to explore chemical reactivity, anticipate environmental impacts, and innovate toward cleaner industrial processes.
The beauty of SO₂ lies not only in its simplicity but in the rich, measurable complexity encoded in its atomic arrangement—one that continues to inform research, policy, and public understanding of chemistry’s role in the world.

Related Post

Mastering The SO₂ Lewis Structure: Step-by-Step Mastery With Visuals and Science

Streamline Your Coverage: Master Toyota Insurance Easy Login and Quick Registration with Confidence

