Protons, Neutrons, and Electrons: The Atomic Heartbeat of Hydrogen
Protons, Neutrons, and Electrons: The Atomic Heartbeat of Hydrogen
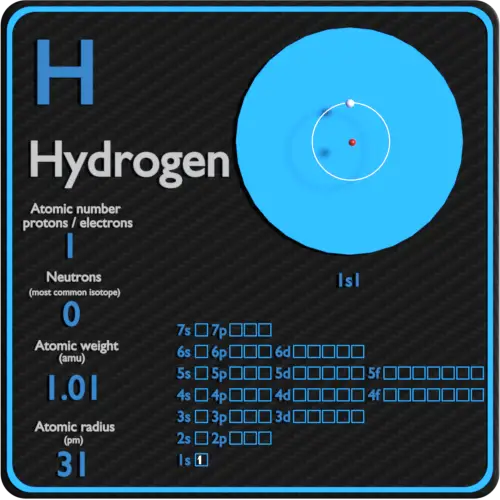
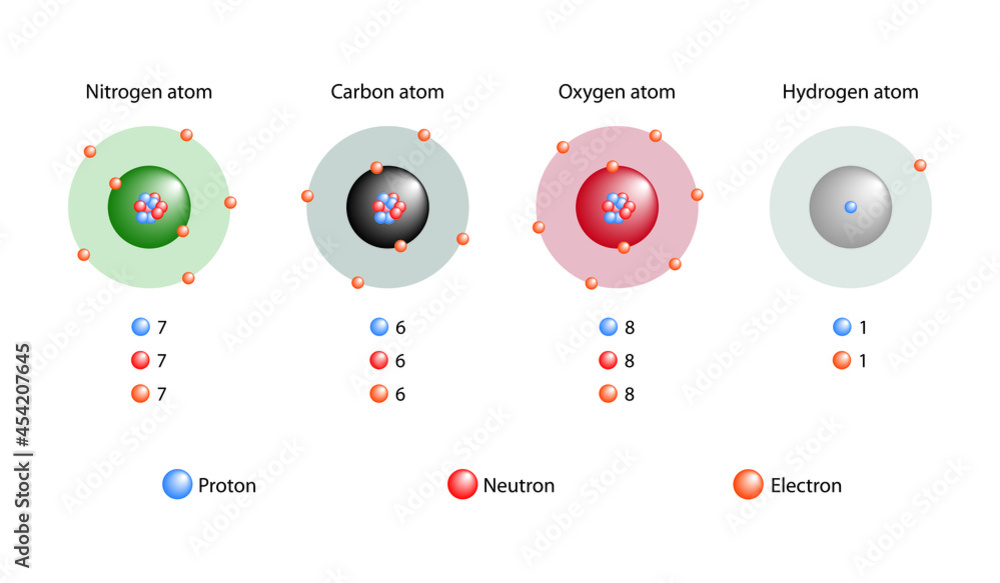
Hydrogen, the simplest and most abundant element in the universe, reveals its profound significance through its fundamental substructure—protons, neutrons, and electrons. Comprising just one proton, one electron, and typically noneutron, hydrogen’s atomic identity lies in this tiny trio, setting the stage for its pivotal role in chemistry, astrophysics, and renewable energy. Understanding the precise composition of hydrogen reveals not just its identity—but how it powers stars, fuels innovation, and shapes the natural world.
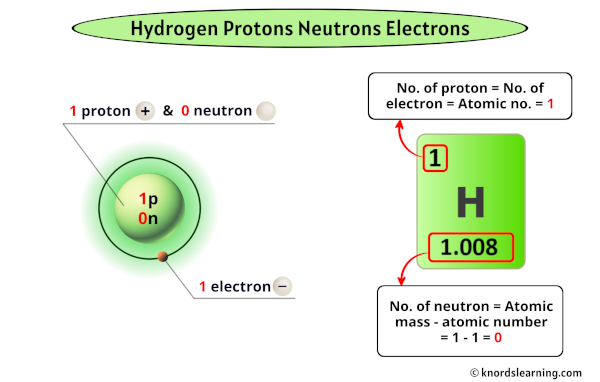
The proton, a positively charged subatomic particle, defines hydrogen’s atomic number. With a mass of 1 atomic mass unit (amu) and a charge of +1 elementary charge, the proton is the defining core of the hydrogen atom. This single proton resides in the nucleus, exerting the electromagnetic force that binds the electron in a delicate orbital dance.
Hydrogen is unique among elements by having only one proton; any deviation—such as a second neutron forming deuterium—alters the element entirely. “Hydrogen is the elemental blueprint of astrophysics,” notes physicist Dr. Elena Vasquez, “because it is the primary fuel of stars, and its simplicity belies cosmic importance.”
The neutron, a neutral particle found in the nucleus, fundamentally distinguishes common hydrogen from its heavier isotopes.
A standard hydrogen atom contains zero neutrons—making it protium, the most prevalent isotope. Yet, the presence of a neutron transforms hydrogen into deuterium, a heavy isotope with a single proton and one neutron. This difference, though subtle, influences nuclear reactions and phase behavior.
In nuclear fusion reactions powering stars, the neutron’s role becomes critical—though in pure hydrogen processes, the proton alone governs atomic stability and electron interactions. “The neutron adds complexity but also a window into stellar nucleosynthesis,” explains nuclear chemist Dr. Rajani Mehta.
“Without neutrons, hydrogen’s role in fusion would be nonexistent, yet its basic atomic behavior hinges solely on the proton’s presence.”
Electrons complete hydrogen’s atomic triad, orbiting the nucleus in stable energy levels governed by quantum mechanics. With a negative charge of precisely −1 elementary charge, the electron balances the proton’s positive charge, creating a neutral atom. Its movement fuels chemical reactivity and energy transitions, determining how hydrogen bonds, fuels reactions, and interacts across scales.
“An electron in hydrogen occupies the first energy level—originally predicted by Niels Bohr—allowing precise modeling of electron transitions, Ionization energies, and bond formation,” says Dr. Vasquez. “Even a single electron gives hydrogen its defining chemical identity.”
This trio—proton, neutron (when present), and electron—defines hydrogen’s atomic foundation.
In its purest form, hydrogen exists as protium, a single-proton atom with electron bonding that enables polyvalent chemistry. Beyond isotopes, the proton remains constant: a threshold of identity. “Hydrogen’s power lies in its elemental simplicity,” asserts Dr.
Mehta. “One proton, one electron—these are the minimalists of the atomic world, yet they drive immense physical and chemical phenomena across laboratories and stars.”
While deuterium and tritium offer isotopic variants with neutron’s addition, protium, powered solely by its core proton and electron, remains the dominant isotope—constituting about 99.98% of natural hydrogen. This stability enables reliable use in fuel cells, fusion research, and spectroscopy.
“The single proton in hydrogen’s nucleus provides both identity and functionality,” highlights Dr. Vasquez. “Its presence ensures hydrogen’s role as a fundamental building block of matter and energy.”
Hydrogen’s atomic structure, though elementary, underpins vast swathes of scientific inquiry and technological advance.
From stellar fusion where proton-proton chains forge energy, to cutting-edge hydrogen economy initiatives, the interplay of protons, neutrons, and electrons defines its versatility and centrality. “Hydrogen is not just an atom—it’s the cornerstone of matter’s reactivity and energy potential,” concludes Dr. Mehta.
“At its core, every atom of hydrogen carries one proton, and only one electron in its most stable state—a trinity that powers the universe’s simplest yet most profound elements.”

Related Post
Who Is Kiyamma Griffin Chyna Tahjere Griffins Father and Faith Evans Baby Daddy
University St Augustine: A Legacy of Learning Woven Through History and Innovation

Leaked Snaps Expose Charlotte Parkes of Emerge: A Glimpse Behind the Public Persona
Elise Menaker Marquee Sports Network Bio Wiki Age Height Family Husband Salary Net Worth

