Power at the Cellular Level: The Core Reactants and Products of Respiration
Power at the Cellular Level: The Core Reactants and Products of Respiration
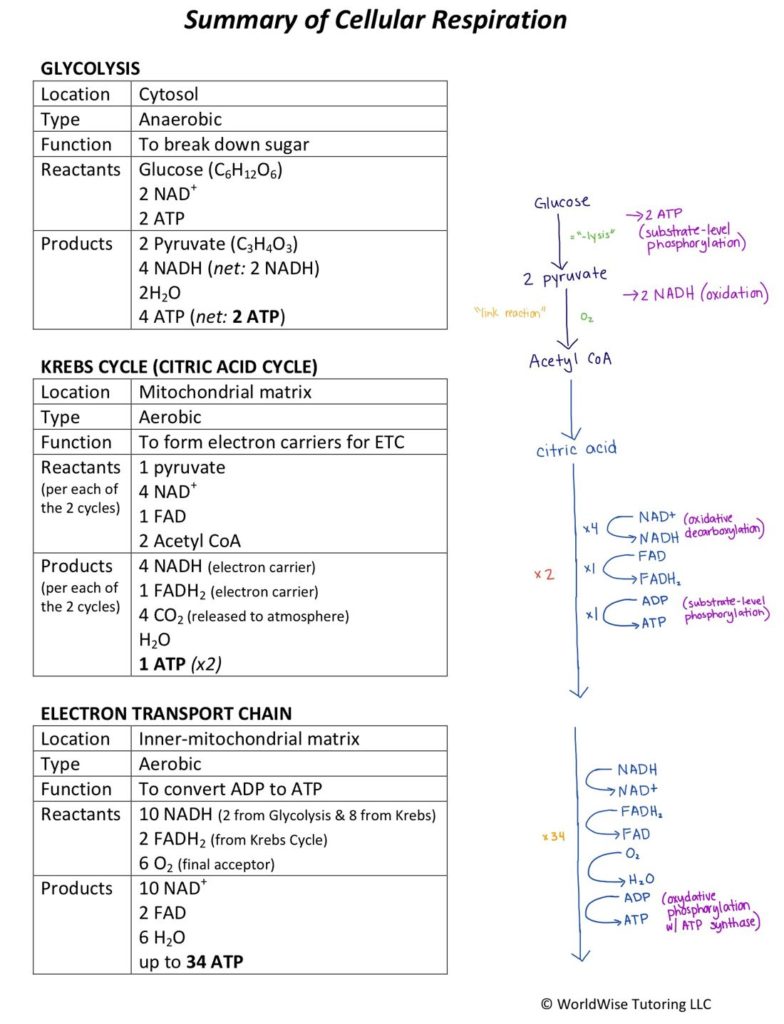
Reactants and products of cellular respiration form the biochemical backbone of life’s energy conversion process, enabling cells to transform stored molecular energy into usable fuel. This intricate metabolic pathway converts glucose and oxygen into adenosine triphosphate (ATP), the universal energy currency of cells. The process is far from a single step; it unfolds through a series of carefully orchestrated reactions, each pivotal in sustaining life at the smallest biological scale.
Exploding the Chemical Equation: Reactants Driving Energy Release
At the heart of cellular respiration are three primary reactants: glucose (C₆H₁₂O₆), oxygen (O₂), and, crucially, the cellular machinery embedded in mitochondria that powers the transformations. Glucose serves as the fuel—an energy-dense sugar derived from dietary carbohydrates or stored glycogen. Its breakdown begins in the cytoplasm during glycolysis, where a single six-carbon molecule splits into two three-carbon pyruvate units, releasing a modest 2 molecules of ATP and generating NADH, an electron carrier loaded with high-energy electrons.Oxygen acts as the final electron acceptor in the electron transport chain, a sequence of protein complexes embedded in the inner mitochondrial membrane. Without oxygen, this chain collapses, halting ATP production and forcing cells into anaerobic pathways. Thus, oxygen is not a mere participant—it is essential for maximizing energy harvest.
Other reactants include inorganic phosphate (Pi), required for ATP synthase to generate ATP, and water (H₂O), formed when oxygen combines with electrons and protons at complex IV.
ATP: The Vital Product Powering millions of Cellular Functions
The most significant product of cellular respiration is adenosine triphosphate (ATP), synthesized through oxidative phosphorylation in the mitochondria. For every complete oxidation of one glucose molecule, approximately 30–32 ATP molecules are formed.The产出 follows a precise biochemical pathway: - **Glycolysis** produces 2 ATP (net) and 2 NADH. - **Krebs cycle** generates 2 ATP per glucose, along with 6 NADH and 2 FADH₂. - **Electron transport chain** uses NADH and FADH₂ to pump protons across the inner mitochondrial membrane, creating a gradient that drives ATP synthase.
This ATP fuels nearly every cellular process: muscle contraction, nerve impulse conduction, protein synthesis, and active transport. “Nearly every energy-requiring function in eukaryotic cells depends on ATP derived from respiration,” asserts Mark W. Nelson, biochemistry professor at Stanford.
“Without this molecular battery, life as we know it would not exist.”
Beyond ATP, cellular respiration yields two critical byproducts: carbon dioxide (CO₂) and water (H₂O). Carbon dioxide, a waste product of glucose oxidation, diffuses out of mitochondria into the bloodstream, where it is transported to the lungs and exhaled—“a silent but essential release that maintains pH balance and respiratory homeostasis,” explains Dr. Elena Torres, cellular metabolism specialist.
Water, formed at the final stage of the electron transport chain when oxygen accepts electrons and protons, is itself vital.
This hydrogen byproduct combines with hydrogen ions to form H₂O, which not only sustains internal hydration but also supports hydrolytic metabolic reactions.
Efficiency and Variability: Adaptations in Energy Capture
Cells adapt variably depending on oxygen availability and metabolic demands. While aerobic respiration delivers maximal ATP, some tissues can switch to anaerobic glycolysis—fermentation—producing just 2 ATP per glucose, but rapidly under low-oxygen conditions. In metazoans like humans, aerobic metabolism reigns, but certain microbes thrive anaerobically, using alternative electron acceptors such as sulfate or nitrate.Moreover, mitochondria themselves exhibit dynamic control: regulated by cellular energy status via enzymes like pyruvate dehydrogenase and ATP synthase. This feedback ensures energy production matches demand, preventing wasteful overproduction.
Understanding the reactants and products of cellular respiration reveals not just a chemical equation, but a marvel of biological engineering—efficient, adaptable, and essential.
From glucose to ATP, oxygen to water, each molecule plays a deliberate role in sustaining life’s energy economy, a process honed over billions of years of evolution.
As research advances in metabolic diseases and energy metabolism, mapping these core components remains foundational—illuminating pathways from cellular function to therapeutic targets for conditions ranging from cancer to neurodegenerative disorders. In every breath and every cell, the dance of reactants and products continues silently, relentlessly fueling life itself.



Related Post
Navigating the Realm of Hindimovie2: A Exhaustive Review

The Dark Truth: Paul Walker’s Disturbing Secret Revealed

Did Shawn From FGTev Die? Unraveling the Truth Behind the Rumors

