N2O vs. NO2: The Molecular Showdown That Shapes Our Atmosphere
N2O vs. NO2: The Molecular Showdown That Shapes Our Atmosphere
In the complex theater of atmospheric chemistry, two nitrogen oxides—N2O (nitrous oxide) and NO₂ (nitrogen dioxide)—play distinct roles with profound implications for climate, air quality, and human health. Though both contain nitrogen and oxygen, their chemical structures, reactivities, and environmental impacts diverge dramatically. Understanding the nuanced differences between N₂O and NO₂ is not merely academic—it is essential to addressing global challenges like ozone depletion, greenhouse gas management, and urban pollution.
This deep dive unpacks their molecular architecture, reactivity profiles, and real-world consequences with clarity and precision.
Molecular Structure and Bonding: How Subtle Differences Create Distinct Behaviors
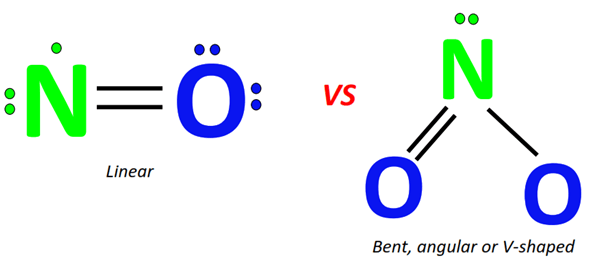
At the core, N₂O and NO₂ differ fundamentally in their atomic composition and bonding patterns. Nitrous oxide (N₂O), often called “laughing gas,” is a linear, symmetric molecule consisting of two nitrogen atoms flanking a single oxygen atom (N–N–O).The N–N bond is short and strong, with a C–N equivalent energy, contributing to N₂O’s remarkable stability under ambient conditions. Its dipole moment is low—much smaller than NO₂—due to balanced charge distribution across its symmetric structure. NO₂, by contrast, is a bent, asymmetric molecule where a nitrogen atom forms double bonds with one oxygen and a single bond with a second oxygen (N=O–O).
This structural asymmetry generates a significant dipole moment, making NO₂ polar and highly reactive. The presence of unpaired electrons on the central nitrogen atom imparts unique electronic properties: NO₂ acts as a strong oxidizing agent, readily participating in radical-driven redox reactions. Dr.
Elena Rostova, a physical chemist specializing in atmospheric species, explains: “N₂O’s symmetric architecture shields its nitrogen-oxygen bonds from immediate attack, rendering it inert to many reactive pathways. NO₂, however, reacts vigorously due to its bent geometry and uneven electron distribution—this is what makes it so chemically aggressive.”
Physical and Chemical Properties: Stability, Reactivity, and Solubility
Surface properties reveal striking differences between the two compounds. N₂O remains a colorless, non-toxic gas at room temperature with a boiling point of −89.4 °C and moderate volatility.Its solubility in water is limited—about 10 mg/L at 20 °C—meaning it resists rapid dissolution and persists longer in the atmosphere. NO₂ presents a sharp contrast: highly colored (rust-red in light), toxic, and vastly more soluble in water—solubility exceeds 20 mg/L at 20 °C—facilitating its dissolution in respiratory tracts and moisture-rich atmospheric aerosols. Its reactivity is equally pronounced: NO₂ undergoes rapid photolysis under sunlight, breaking into nitric oxide (NO) and an oxygen atom.
This initiates cascades of atmospheric chemistry, most notably the destruction of stratospheric ozone. N₂O, by comparison, resists photolysis and persists in the troposphere for over a century, acting as a potent long-lived greenhouse gas. Its relatively low reactivity allows it to accumulate and migrate upward, where eventual oxidation converts it into NOx—indirectly contributing to ozone layer depletion far from its emission source.
Environmental solubility also dictates how each species interacts with ecosystems. NO₂ readily dissolves into rain, forming nitric acid and contributing significantly to acid deposition, while N₂O passes largely unaltered through the lower atmosphere, only decomposing at high altitudes or in the stratosphere.
Environmental and Health Impacts: From Climate Forcing to Respiratory Risk
The divergent behaviors of N₂O and NO₂ manifest in distinct environmental and health consequences.N₂O is a key player in global warming, with a global warming potential (GWP) 273 times that of CO₂ over a 100-year horizon. Though emitted in lower volumes—approximately 15 million metric tons annually—the species’ longevity amplifies its cumulative effect. As noted by the Intergovernmental Panel on Climate Change (IPCC), N₂O accounts for roughly 6% of total anthropogenic greenhouse gas forcing.
NO₂, while shorter-lived (half-life ~30 hours), exerts acute impacts on human health and air quality. As a primary component of traffic-related smog, it irritates airway epithelia, exacerbates asthma, and increases susceptibility to respiratory infections. Long-term exposure correlates with reduced lung function and heightened cardiovascular risks, particularly in urban centers.
Beyond health, NO₂ serves as a critical precursor to ground-level ozone and fine particulate matter (PM₂.₅), both classified as hazardous air pollutants. Regulatory agencies like the U.S. EPA enforce strict NO₂ limits due to its proven contribution to smog formation and ecosystem stress.
N₂O’s environmental toll extends beyond climate. When ascending into the stratosphere, it triggers O₃ destruction via catalytic cycles involving nitrogen oxides—each N₂O molecule capable of destroying thousands of ozone molecules over decades. Though present in the lower atmosphere, its long atmospheric lifetime ensures eventual global impact.
Scientists emphasize that tracking both species is essential for effective mitigation. As Dr. Jonathan Kim, an atmospheric chemist, notes: “N₂O isn’t just a passenger in climate change—it’s a driver, quietly accumulating with decades-long consequences.
NO₂, reactive and immediate, demands real-time monitoring in polluted cities. Both demand attention—but their roles are fundamentally different.
Industrial Roles and Emerging Applications: Beyond Emissions
Beyond their atmospheric roles, N₂O and NO₂ serve critical functions in industrial chemistry. N₂O, valued for its thermal and chemical stability, functions as a refrigerant in cryogenics, a propellant in aerospace systems, and—as a precursor—in the synthesis of nitric acid and pharmaceuticals like sulfa drugs.Its use in surgical anesthesia, though diminishing, remains recognized for its low toxicity and rapid recovery profile. NO₂, though primarily an industrial byproduct, plays a dual role. In the chemical industry, it serves as a key oxidant in the production of nitric acid (via the Ostwald process), essential for fertilizers and explosives.
It also finds use as a bleaching agent and disinfection precursor, though its aggressiveness limits direct health applications. “Innovations now seek to repurpose these molecules beyond pollutant status,” says Dr. Amina Patel, a chemical engineer focused on green chemistry.
“N₂O’s stability inspires safer long-halopolitan alternatives, while NO₂’s reactivity guides smarter catalytic systems—potentially enabling greener synthetic routes.” Emerging research explores catalytic conversion of NO₂ into less harmful species using advanced nanomaterials, aiming to mitigate urban NO₂ pollution. For N₂O, strategies to reduce emissions focus on agricultural optimization, as fertilizers remain the dominant source, and industrial process redesigns to minimize release during synthesis.
These adaptive pathways underscore a pivotal truth: while N₂O and NO₂ differ in structure and reactivity, their fates in the environment—and responses to human intervention—demand tailored, science-driven management.
The contrast between N₂O and NO₂ illustrates chemistry’s dual nature: subtle atomic differences yield profound environmental and public health outcomes.
N₂O, a silent, persistent greenhouse gas with long atmospheric reach, underscores the urgency of addressing legacy emissions and future accumulation. NO₂, reactive, toxic, and immediate, demands real-time air quality control and targeted pollution abatement. Understanding these roles enables precise policy, innovation, and stewardship—transforming molecular insights into meaningful action.

![Molecular Geometry of NO2- [with video and free study guide]](https://www.aceorganicchem.com/blog/wp-content/uploads/2023/05/VSEPR-replaement-chart.jpg)
![Molecular Geometry of NO2- [with video and free study guide]](https://www.aceorganicchem.com/blog/wp-content/uploads/2023/05/VSEPR-geomtries-chart.jpg)
Related Post

Brandon Jack James: The Rising Intellectual Force Redefining Modern Discourse

The Wolf Of Wall Street Cast: The Wild Rituals and Real Stories Behind Leonardo DiCaprio’s Iconic Role
Investigating the Essential Forensic Struggle of Francine Lymon: Establishing Doo-Wop Copyright Possession

