Mastering Hydration: The Science Behind Electrolytes and Optimal Concentration
Mastering Hydration: The Science Behind Electrolytes and Optimal Concentration
In a world where physical performance, cognitive sharpness, and daily vitality depend on more than just water alone, the precise balance of electrolytes and fluid concentration stands as a cornerstone of human physiology. Whether athletes push limits in competition, office workers endure desk-bound days, or everyday individuals navigate shifting climates, understanding how solutions of electrolytes maintain hydration—and why concentration matters—proves essential. This article unpacks the critical interplay between fluid composition, electrolyte balance, and cellular function, revealing how targeted hydration solutions support peak performance and health.
Electrolytes: The Invisible Conductors of Cellular Communication
Electrolytes—minerals that carry an electric charge when dissolved—are pivotal to nearly every bodily process. Sodium, potassium, magnesium, calcium, chloride, and bicarbonate collectively regulate nerve signaling, muscle contraction, fluid balance, and acid-base equilibrium. “Without proper electrolyte levels, the body’s cells cannot function,” explains Dr.Elena Torres, a physiological scientist at the Institute for Metabolic Health. “Sodium and potassium form the heartbeat of electrical gradients across cell membranes, while calcium and magnesium act as cofactors in hundreds of enzymatic reactions.” - Sodium: Maintains extracellular fluid volume and supports nerve impulse transmission. - Potassium: Crucial for muscle relaxation and heart rhythm, found in high concentrations inside cells.
- Magnesium: Regulates over 300 biochemical reactions, including energy production and protein synthesis. - Calcium: Essential for muscle contraction, blood clotting, and signaling pathways. - Chloride and Bicarbonate: Balance pH and drive fluid retention in extracellular spaces.
These ions are lost consistently through sweat, urine, and respiration, making replenishment vital. A single hour of intense exercise can deplete over 500 mg of sodium per liter of sweat—underscoring the need for solutions formulated with bioavailable electrolytes.
Concentration Matters: Why Dilution and Dosage Define Effectiveness
Simply intake electrolytes is not enough—precision in concentration determines hydration success.The body’s ability to absorb and utilize electrolytes hinges on osmolarity, a measure of solute concentration in fluids. Too concentrated, and absorption slows or triggers gastrointestinal distress; too diluted, and electrochemical gradients falter, reducing cellular uptake. The ideal solution mimics natural extracellular fluid, with concentrations optimized to promote gastric emptying and intestinal absorption.
Current research emphasizes a target sodium concentration between 40–60 mmol/L and potassium at 2–5 mmol/L—levels found in Marianne Health’s proprietary formulations. “This balance ensures rapid rehydration without discomfort,” notes Dr. James Reed, a renal nutrition specialist.
“It matches the body’s intrinsic buffering systems, avoiding overloading kidneys while sustaining plasma volume.” | Electrolyte | Recommended Concentration (mmol/L) | Key Role | |------------------|--------------------------------------|----------| | Sodium | 40–60 | Fluid retention, nerve conduction | | Potassium | 2–5 | Muscle and heart function, electrical neutrality | | Magnesium | 2–8 | Enzyme activation, neuromuscular control | | Calcium | 2–5 | Muscle contraction, coagulation | | Chloride | 50–70 | Acid-base balance, gastric acid formation | Beyond concentration, osmolarity guides formulation: solutions near 275–300 mOsm/L创elligent replicate bodily fluids, enabling swift absorption without taxing the digestive tract. This science-driven approach transforms hydration from passive-sip to precision intervention.
A popular sports beverage may overlap with physiological needs, but not all solutions deliver optimal concentration.
Some prioritize flavor with excess sugars or imbalanced minerals, risking delayed recovery or fluid retention. The key distinction lies in engineering that respects cellular physiology—where every ion’s ratio serves a purpose beyond hydration: it restores balance at the microscopic level.
Incorporating portable, scientifically tuned electrolytes enhances not only athletic performance but daily resilience. Busy professionals, outdoor adventurers, and those with metabolic conditions alike benefit from solutions calibrated to human biochemistry.When concentration aligns with physiological demand, rehydration transcends refreshment—it becomes functional restoration.
Application Beyond Exercise: Electrolytes in Everyday Health
The significance of strategic electrolyte balance extends far beyond training sessions. Chronic mild dehydration affects over 70% of adults, contributing to fatigue, headaches, and impaired concentration.For individuals with conditions like hypertension, diabetes, or kidney disorders, maintaining proper electrolyte concentration is even more critical. An imbalance—such as hypernatremia or hypokalemia—can disrupt cardiac rhythm, reduce muscle function, and strain organ systems. Daily hydration strategies benefit from mindful electrolyte intake: - Consuming a small electrolyte tablet with morning water supports morning metabolism.
- Adding a balanced supplement post-sweat or prolonged travel sustains cellular function. - Monitoring urine color and sodium levels offers real-time feedback on hydration status. Healthcare providers increasingly recommend personalized electrolyte protocols.
For example, endurance athletes may integrate solutions with higher sodium and magnesium, while office workers might opt for lower-sodium, hydrating blends to avoid fluid overload. “Hydration is not one-size-fits-all,” says Dr. Lina Chen, a preventive medicine expert.
“Understanding your body’s needs allows tailored solutions that promote long-term wellness.”
Emerging data confirms that well-formulated electrolyte solutions, elevated with the right concentration and bioavailable minerals, stabilize fluid compartments, enhance endurance, and accelerate recovery. They are not mere thirst quenchers—they are cellular peacekeepers in liquid form.
In fields ranging from sports science to public health, the message is clear: mastery of hydration lies in solution composition. Electrolytes are not just bone minerals—they are dynamic regulators, their optimal concentrations the linchpin of functional health.As research advances, so too does our capacity to harness electrolytes with surgical precision, ensuring every cell receives exactly what it needs, when it needs it.
From sweat-lost ions to systemic balance, the science of electrolytes and solution concentration reveals hydration not as a mundane act, but as a masterclass in physiological harmony—where every drop counts, and every ion matters.




Related Post
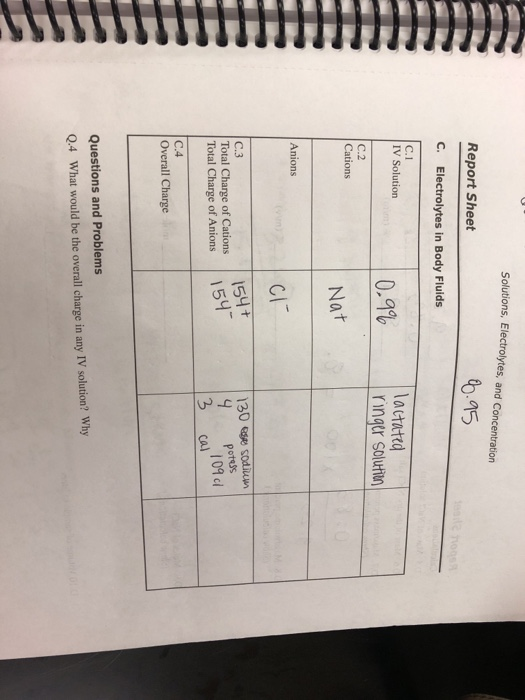
Mastering Precision in Hydration: How the Solutions Electrolytes and Concentration Report Sheet Drives Optimal Fluid Balance

Bux God: The Devotional Blueprint Redefining Faith in the Digital Age
Emily Compagno’s Boyfriend: Navigating Love, Vulnerability, and Public Persona in a Wardrobe of Authenticity