Mastering Coulombic Attraction: The Core of Electrostatic Forces in Chemistry
Mastering Coulombic Attraction: The Core of Electrostatic Forces in Chemistry
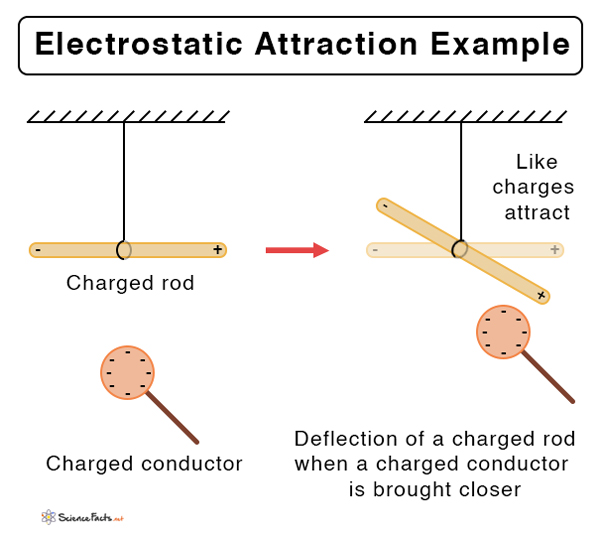
At the heart of atomic interactions lies Coulombic attraction—the fundamental electrostatic force that governs how charged particles influence one another. This principle, explored deeply in the Pulgar Answers Key (Pogil) module, reveals that opposite charges attract while like charges repel, dictating everything from molecular bonding to ionic crystal structures. Understanding Coulombic attraction is not just academic—it underpins modern chemistry, materials science, and biological systems alike.
Central to this concept is Coulomb’s Law, mathematically expressed as F ∝ q₁q₂/r², where force (F) depends on the product of the charges (q₁ and q₂) and inversely on the square of their separation (r). The podcast-style Pogil answers emphasize this proportional relationship, helping students grasp how even minute changes in charge or distance dramatically alter electrostatic forces. For example, doubling one charge while halving the distance between particles quadruples the force—demonstrating the sensitivity of Coulombic interactions.
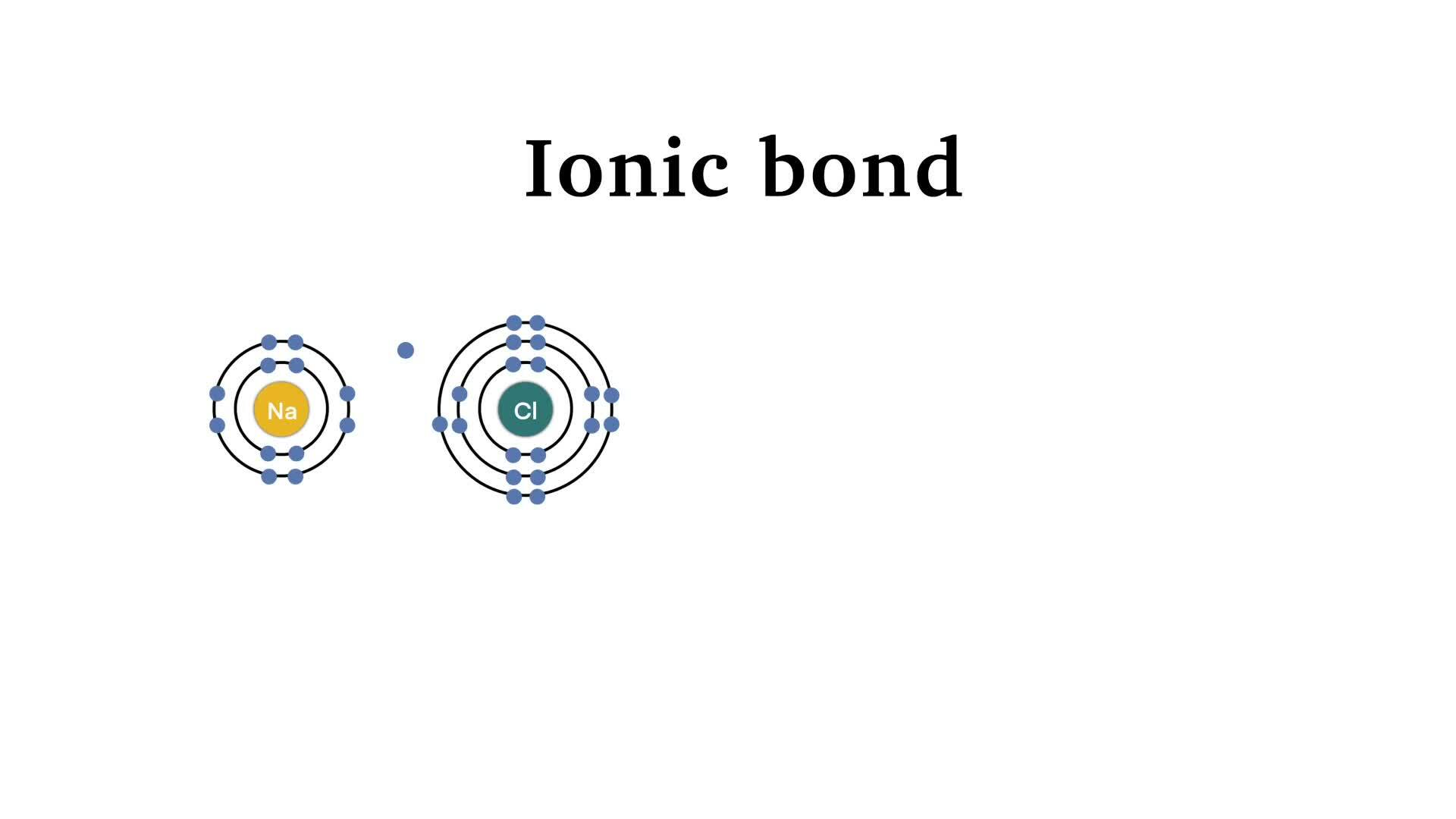
In chemical systems, Coulombic attraction drives electron transfer between atoms, forming ionic bonds when electrons move from electronegative to electropositive species. Consider sodium chloride: sodium donates a +1 charge and chlorine a −1 charge. Their mutual attraction—rooted in Coulombic forces—stabilizes the crystal lattice, creating a solid at room temperature with high melting points.
This process is visualized in Pogil exercises through charged ball-and-stick models and force diagrams, reinforcing the connection between charge, distance, and bond strength.
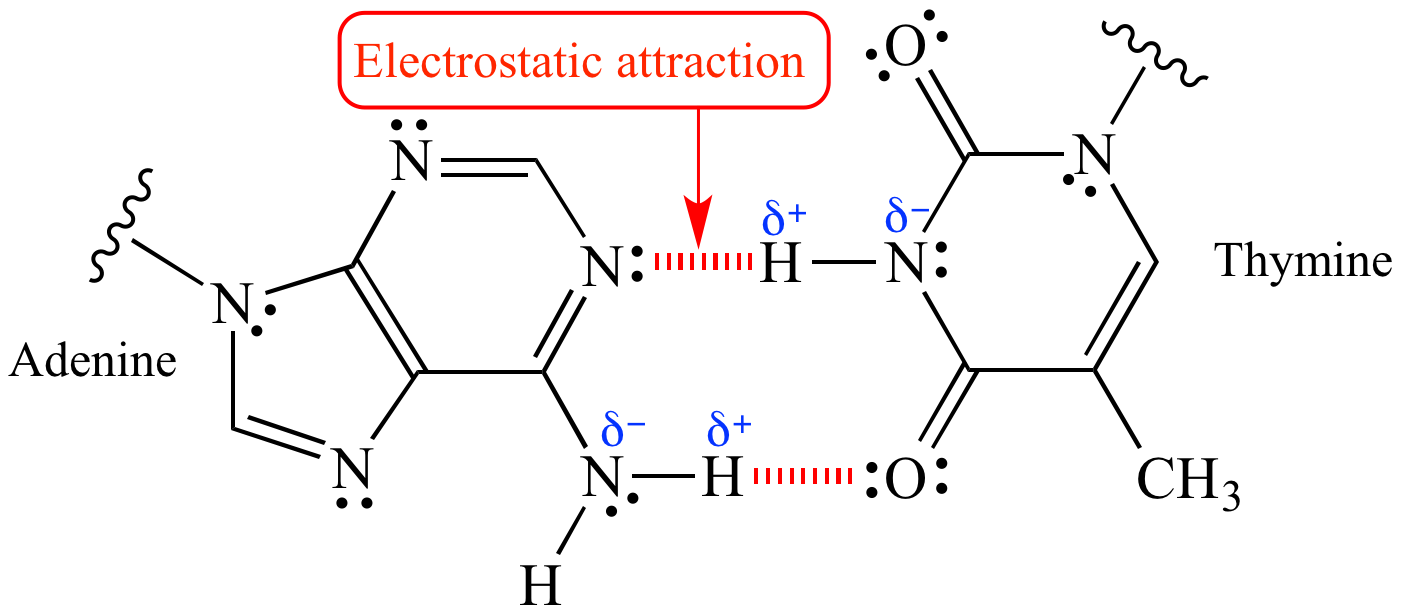
Beyond ionic compounds, Coulombic attraction shapes covalent bonding dynamics as well. Though covalent bonds involve shared electrons, instantaneous polarization and charge separation induce dipole moments—microscopic attractions governed by Coulombic principles. The Pogil framework highlights how these forces influence molecular geometry, polarity, and intermolecular interactions such as hydrogen bonding and van der Waals forces.
Key Variables in Coulombic Attraction
- Charge Magnitude: Larger charges produce stronger attractions or repulsions, directly scaling the force per Coulomb’s Law.- Inverse Square Distance: Force diminishes rapidly as separation increases—halving distance triples the force. - Medium Impact: The dielectric constant of a material modifies effective charge strength; polar solvents weaken Coulombic interactions compared to vacuum, altering bonding behaviors. - Charge Sign: Opposite charges attract, driving fundamental bond formation; like charges repel, maintaining electron equilibrium.
The Pogil Answers Key consistently reinforces these variables through guided inquiry—students analyze real-world systems like acid-base interactions or crystal lattice energies by quantifying charge effects and geometric configurations. This hands-on engagement transforms abstract equations into tangible chemical phenomena.
Educational Applications and Learning Tools
Classroom success with Coulombic attraction hinges on conceptual clarity—Pogil’s approach bridges math, physics, and chemistry by embedding equations in molecular contexts. Example problems show students calculating force changes across different ionic radii or predicting bond stability from charge distribution.
Visual simulations model how increasing ion concentration in electrolytes enhances electrical conductivity, directly linking Coulombic forces to macroscopic behavior.
Quote from Pogil Frameworks: “Electrostatic forces are invisible yet indispensable—advanced understanding starts with recognizing how charge and distance orchestrate atomic-scale events.” This insight enables learners to see beyond formulas to the dynamic physical reality beneath chemical forces.
Real-world applications abound: battery design relies on Coulombic energy between ions to store and release power; biological membranes depend on polar charge attractions to guide ion transport across cell walls. Nanomaterials and catalysts leverage engineered Coulombic environments to enhance reactivity and efficiency. Each example roots itself in the foundational law Bob Coulomb first described centuries ago, now refined by modern physics and molecular theory.
Comprehending Coulombic attraction is essential not only for chemists but for engineers, biologists, and materials scientists. It forms the invisible scaffold upon which matter’s complexity is built—from crystal lattices to enzymatic active sites. The Pogil curriculum transforms this complexity into understanding by guiding students through problem-solving that integrates theory with measurable outcomes, making abstract forces visible and measurable.
Ultimately, mastery of Coulombic attraction equips learners to predict, explain, and manipulate chemical behavior with precision—turning electrostatic phenomena from abstract principles into tools for innovation. The Pogil Answers Key serves as a vital compass in this journey, offering structured pathways that clarify the central role of attraction in the quiet yet powerful dance of charged particles.

Related Post

Best Karaoke Songs for Men: The Power of Catharsis, Rhythm, and Riffing Fair
Super Sonic Fleetway: The Ultimate Fusion of Speed, Power, and Sonic Innovation
Cody Rhodes Makes Young Fans Day After Finn Balor Tears Up Sign

Who Holds the Money Behind Putin? A Closer Look at the President’s Billion-Dollar Legacy

