Lewis Structure for Potassium: Unlocking the Element’s Atomic Architecture and Chemical Identity
Lewis Structure for Potassium: Unlocking the Element’s Atomic Architecture and Chemical Identity
Potassium, with atomic number 19 and symbol K, stands as a luminous pillar in the periodic table—vital for biological function and indispensable in modern technology. While its soft, silvery-white appearance captivates visually, its electron configuration and bonding behavior, best illuminated through Lewis structure principles, reveal a deeper story of reactivity and atomic organization. Unlike heavier alkali metals, potassium’s electron arrangement governs its nucleophilic character, low ionization energy, and preference for forming strong ionic bonds—especially with halides.
Understanding its Lewis structure is not merely academic; it unlocks insight into its role in everything from nerve transmission to battery innovations. This exploration decodes potassium’s atomic blueprint, guided by the clarity of Lewis notation, to explain how its valence electrons shape chemistry across disciplines.
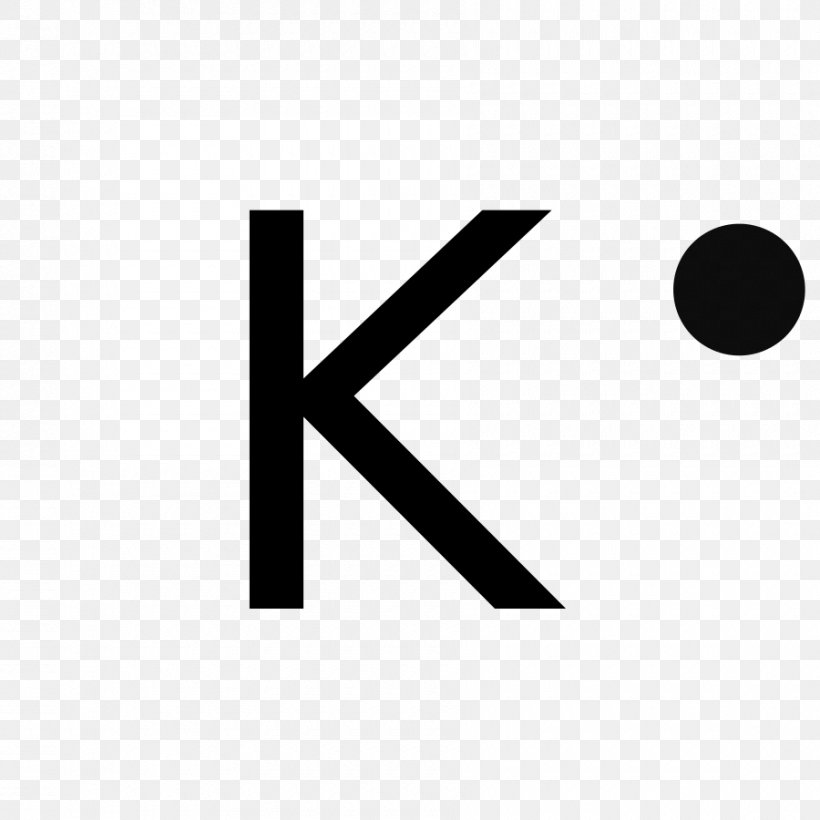
At the heart of computational chemistry lies the Lewis structure—a tool that maps valence electrons to depict an atom’s bonding potential. For potassium, this visualization begins with its atomic electron configuration: 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹.
The outermost shell, defined by the 4s orbital, contains exactly one valence electron—a pivotal detail. “The singular electron in potassium’s 4s orbital drives its remarkable reactivity,” notes chemical structuralist Dr. Elena Torres.
“This lone valence electron is weakly held, making potassium one of the most electropositive elements on Earth.” In Lewis terms, this translates to potassium’s default oxidation state of +1, where it readily donates that one electron to achieve stability akin to noble gases.
Visualizing Potassium’s Bonding: The Lewis Framework
The Lewis structure for a potassium atom centers on its lone 4s electron, but to understand its chemical behavior, one must consider bonding scenarios—especially its common interactions with halogens. Potassium does not typically form covalent bonds; instead, it engages in ionic bonding, shedding its single valence electron to form K⁺ ions. In a compound like potassium chloride (KCl), the Lewis framework highlights this transfer: a potassium atom loses 4s¹ to become K⁺, while a chlorine atom gains that electron to form Cl⁻.
The resulting electrostatic attraction between the positively charged K⁺ and chloride ion is the essence of ionic bonding.
In formal Lewis notation, potassium is depicted with its archetypal group 1 (alkali) symbol, but its structural singularity lies in electron configuration. In KCl, the structure shows K⁺ as a hollow ion—its 4s orbital vacated—and Cl⁻ as fully octet-filled, acquiring a stable 3p⁶ configuration. “This elegant transfer mirrors nature’s drive toward electron delocalization,” observes Dr.
Rajiv Mehta, materials chemist at MIT. “Potassium’s one valence electron becomes nature’s signal for charge neutrality and structural balance.” The simplicity of this model belies its power: it predicts solubility, reactivity trends, and bonding preferences with uncanny accuracy across alkali metals.
The Atomic Blueprint: Valence Electrons and Reactivity
Potassium’s position in Group 1 places it among the most reactive metals, and its Lewis structure courageously reflects this. With only one valence electron, potassium exhibits a pronounced ionization tendency—easily losing that electron to achieve a noble gas configuration.
The corresponding Lewis depiction captures this vulnerability: the isolated 4s electron stands out, stripped from shielding by inner shells, making it exceptionally accessible to electrophiles. “This electron’s high energy relative to inner shells makes potassium docile in chemical terms,” explains synthetic chemist Dr. Linh Nguyen.
“It doesn’t share electrons gently—rather, it frees them when prompted.”
Environmental and industrial contexts further emphasize potassium’s atomic behavior. In soil chemistry, potassium ions (K⁺) are vital for plant osmotic regulation and enzyme activation; their Lewis structure-assumed charge enables efficient transport across cell membranes. In engineering, potassium’s electronic duality—readily ionized yet stable after electron transfer—fuels applications like potassium-ion batteries.
“In next-gen energy storage, potassium’s mono-electronic simplicity reduces material costs while maintaining ion mobility,” notes Dr. Carlos Silva, battery technology lead at Stanford’s Precision Energy Lab. “Its Lewis structure underpins this synergy between atomic theory and real-world innovation.”
Comparative Insights: Potassium vs.
Other Alkali Metals
While all Group 1 elements share a 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹ configuration, potassium’s relative nuclear size and electron configuration set it apart from lithium and sodium. Its single valence electron in a larger 4s shell results in lower ionization energy than lithium, yet slightly higher than sodium’s 3s valence electrons—explaining potassium’s intermediate reactivity. “Lewis structures reveal these subtle energetic gradients,” Mehta clarifies.
“Potassium sits mid-stage in the group’s reactivity curve, a balance between ease of electron loss and kinetic stability.” Bullet points illustrating these distinctions: - Lithium: Smaller ionic radius, higher ionization energy, less reactive - Potassium: Larger 4s orbital, low ionization energy, highly electropositive - Sodium: 3s electron—stable octet, moderate reactivity - Potassium: Optimal balance—accessible valence electron, ideal for ionic compounds like KBr and KNO₃
Practical Applications Rooted in Atomic Structure
From biological systems to industrial processes, potassium’s Lewis-compliant bonding enables transformative applications. In human physiology, potassium ions (K⁺) shuttle across neuronal membranes, driving action potentials—ymmetrically enabled by their charged, ionic character. In agriculture, potassium fertilizers deliver essential nutrients via soluble K⁺ ions, directly sustaining crops.
In technology, potassium compounds power advanced batteries, leveraging ionic mobility and low electronic resistance. “Each application is anchored in potassium’s atomic astronomy,” says battery researcher Dr. Nisha Patel.
“Understanding its Lewis framework isn’t abstract—it’s the blueprint for harnessing its full potential.”
In the end, potassium’s story, as written in its Lewis structure, is one of simplicity and consequence. The single valence electron, the +1 charge, the ionic dance of bond formation—all converge to define a metal vital not only to nature, but to the hum of modern innovation. Far from being a mere footnote in the periodic table, potassium exemplifies how atomic architecture and chemical bonding are inseparable, with Lewis notation standing as both a mirror and a map.
This visualization reveals not just how potassium behaves, but why it matters—a testament to the enduring power of fundamental chemistry.

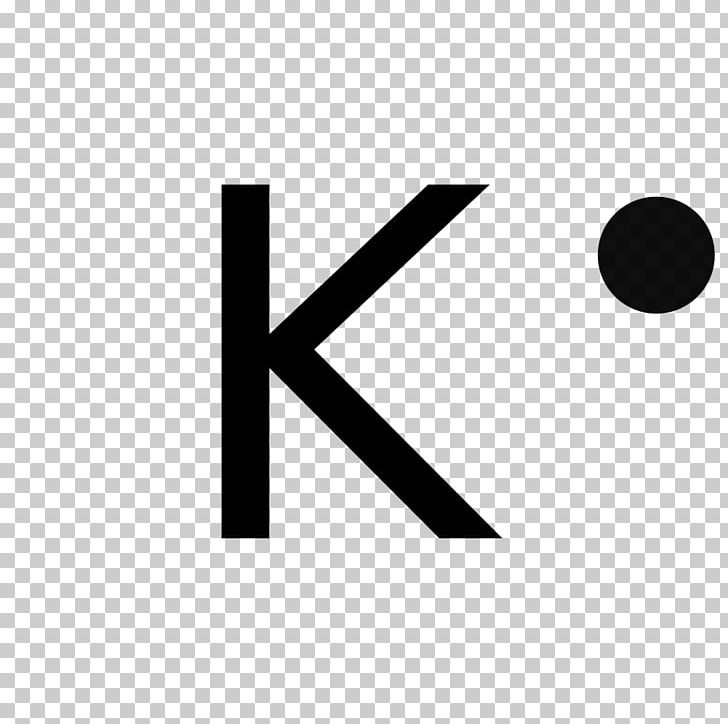

Related Post

PVT vs MCR: Decoding Two Video Game Endangers and the Genres That Define Their Legends

Outsmart Nitrogen’s Silent Power: Decoding the Lewis Dot Diagram Behind the Molecule’s Chemical Soul

Boston Ma Weather Tomorrow: What to Expect in the Next 24 Hours

