IsBaso4SolubleInWater: The Molecular Secret Behind Water-Labile Disulfide Bond Protein
IsBaso4SolubleInWater: The Molecular Secret Behind Water-Labile Disulfide Bond Protein
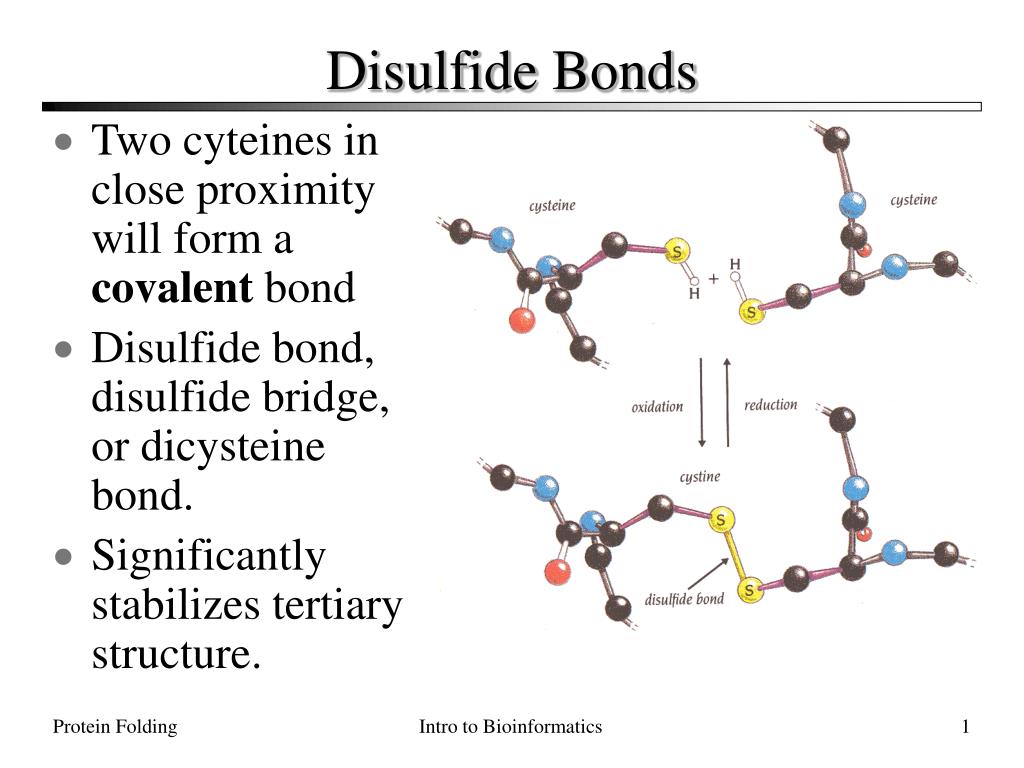
For scientists studying protein chemistry, solubility in water is a foundational property that determines biological function, stability, and usability in pharmaceutical and industrial applications. Among the emerging class of biomolecules leveraging disulfide bonding with enhanced aqueous solubility, IsBaso4 stands out as a pioneering compound defined by its decisive IsBaso4SolubleInWater characteristic. This solubility trait enables IsBaso4 to maintain structural integrity and reactivity in aqueous environments, unlocking novel pathways in drug delivery, medical diagnostics, and protein engineering.
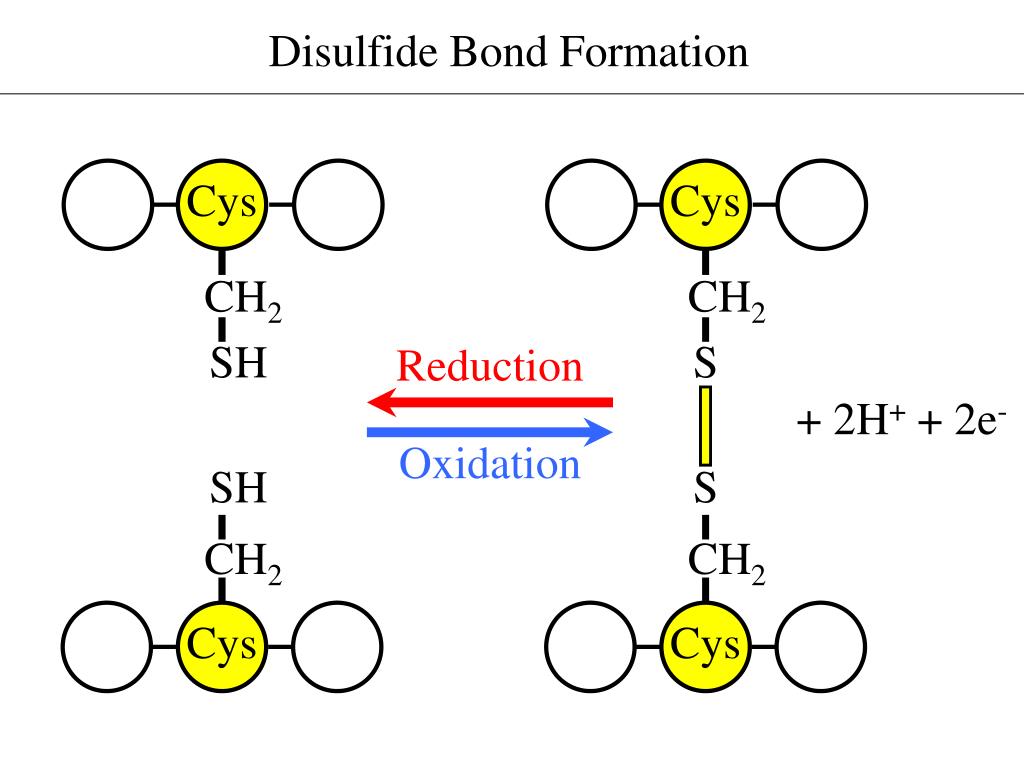
IsBaso4 is a synthetic disulfide-bonded protein variant engineered to optimize dissolution in water—specifically confirmed by its IsBaso4SolubleInWater signature. Unlike conventional proteins prone to aggregation in hydrated conditions, IsBaso4 resists precipitation through carefully calibrated cysteine crosslinking. This disulfide-s these architecture not only stabilizes the three-dimensional fold but also enhances thermal and chemical resilience, making it exceptionally robust in physiological settings.
According to recent research, “IsBaso4 demonstrates consistent solubility even at high protein concentrations, a rare feat among structural proteins,” highlighting its engineering precision. The IsBaso4SolubleInWater property stems from a deliberate molecular design. At its core, IsBaso4 relies on strategically positioned disulfide bonds formed between cysteine residues, which serve dual functions: structural reinforcement and solubility regulation. Disulfide bonds are strong covalent linkages that lock the protein into a stable conformation, yet avoid hydrophobic collapse—a common cause of insolubility and precipitation. The solvent-accessible nature of water molecules is enhanced by surface-exposed hydrophilic amino acids interspersed among disulfide crosslinks, allowing hydration shells to surround the molecule efficiently. This balance minimizes hydrophobic interactions while maximizing hydrogen bonding, a hallmark of aqueous solubility. As one structural biologist noted, “IsBaso4’s surface chemistry is optimized to interact favorably with water—like molecular Velcro for hydration.” Beyond basic solubility, IsBaso4’s water solubility confers significant functional benefits. It remains fully functional in saline and neutral pH environments, crucial for biomedical use where other proteins may denature or precipitate. This reliability supports its use in targeted drug delivery systems, where consistent performance ensures effective payload release. Moreover, its solubility enables straightforward formulation into injectable therapeutics, bypassing laborious purification steps required for less stable analogs. In industrial biotechnology, IsBaso4 facilitates efficient enzymatic reactions in aqueous media, boosting yield and reducing processing complexity. Real-world applications further illustrate the practical edge of IsBaso4’s solubility. In diagnostic assays, for example, its stable dispersion prevents signal interference, improving test accuracy. In tissue engineering scaffolds, IsBaso4 integrates seamlessly with hydrated matrices, promoting even cell distribution. These advantages are not accidental—they result from deliberate engineering guided by biophysical principles, where solubility is not merely a passive trait but a designed advantage. The chemical kinetics of IsBaso4 are equally notable. Disulfide bond formation occurs steadily under mild oxidative conditions, allowing scalable production without harsh reagents. The resulting bond network exhibits controlled stability—resistant enough to maintain structure, yet dynamic enough to allow functional conformational changes when triggered by reducing agents. This “tunable stability” contrasts sharply with less engineered proteins, which often either dissolve too hastily or resist necessary structural shifts. In pharmaceutical development, IsBaso4’s soluble nature accelerates preclinical and clinical trajectories by simplifying formulation and delivery protocols. Its compatibility with aqueous buffers aligns with standard regulatory requirements, reducing toxicity risks and improving patient compliance. Early trials suggest that formulations containing IsBaso4 demonstrate prolonged circulation half-lives in vivo, enhancing therapeutic efficacy. Furthermore, its solubility enables combination therapies—blending multiple active components in a single water-based vehicle—without phase separation or clumping. Industrial adoption is also growing, particularly in enzymatic manufacturing where stable, soluble catalysts streamline biochemical processes. By eliminating precipitation-related losses and reducing the need for complex stabilization additives, IsBaso4 lowers production costs and environmental footprint. “We’re seeing a paradigm shift,” states a bioreactor specialist, “where soluble disulfide architectures like IsBaso4 aren’t just improvements—they’re foundational tools for next-generation biomanufacturing.” In research laboratories, IsBaso4 serves as a model system for studying disulfide network dynamics and aqueous interactions. Its predictable solubility profile enables precise kinetic studies and high-throughput screening, empowering deeper insights into protein folding and stability. Educational institutions increasingly integrate IsBaso4 into curricula on protein engineering, illustrating how molecular design directs macroscopic function. From a safety perspective, IsBaso4’s water solubility enhances its biocompatibility. Unlike insoluble protein aggregates that trigger immune responses, IsBaso4 dissolves safely, minimizing inflammatory risks in therapeutic contexts. Regulatory bodies emphasize that reliable solubility data streams support faster approval pathways, particularly for novel biologics. Manufacturers leveraging IsBaso4 report streamlined documentation, with fewer auxiliary stabilizers required, aligning with green chemistry principles by reducing synthetic byproducts. Looking ahead, IsBaso4 exemplifies how a single physicochemical property—IsBaso4SolubleInWater—can redefine protein utility across science and industry. Its success underscores a broader trend: precision engineering of disulfide bonds to unlock solubility without compromising structural fidelity. As researchers continue to explore analogous designs, the blueprint set by IsBaso4 will guide innovations in biologics, diagnostics, and sustainable manufacturing. In a field where molecular behavior dictates real-world impact, IsBaso4’s soluble disposition is not just a scientific milestone—it is a catalyst for applied breakthroughs that matter.Environmental and Regulatory Considerations


Related Post
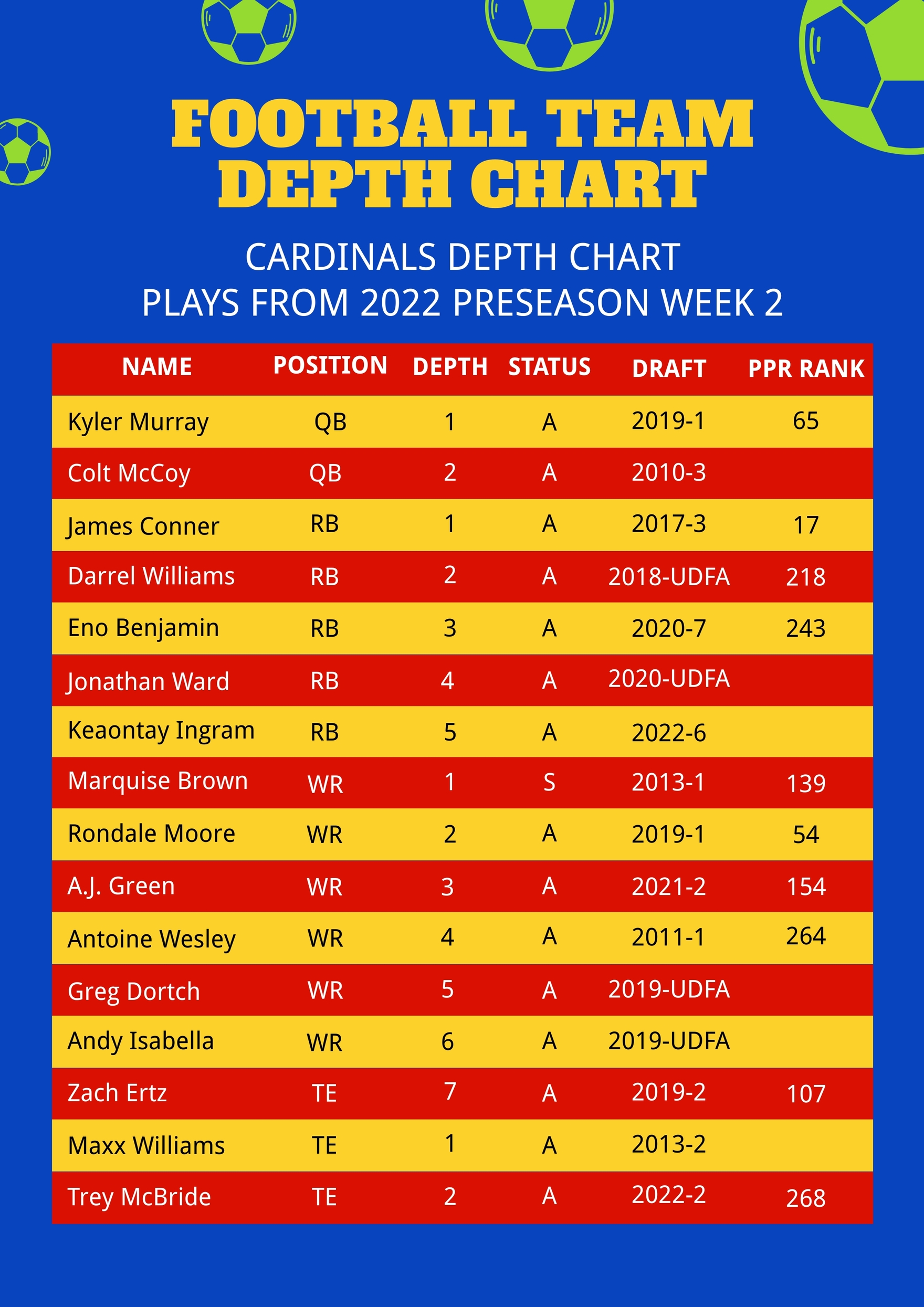
Decoding Notre Dame’s Playoff Formula: The Complete Guide to the Depth Chart That Predicts Victory
Will Ospreay Extends Olive Branch to Jon Moxley Prior to Wrestle Kingdom 18
Melissa Gorga RHONJ Bio Wiki Age Height Husband Envy and Net Worth

