Is the Anomeric Carbon Always Carbon 1? Unlocking the Core of Sugar Chemistry
Is the Anomeric Carbon Always Carbon 1? Unlocking the Core of Sugar Chemistry
For decades, chemists have operated under a foundational assumption in carbohydrate structure: the anomeric carbon is universally regarded as carbon 1. But is this true in every case, or do exceptions reveal deeper complexity in sugar stereochemistry? The answer lies not in a simple yes or no, but in a nuanced exploration of anomeric configurations, epimerization, and the structural dynamics of monosaccharides.
Understanding whether the anomeric carbon is consistently carbon 1 demands a deep dive into the stereochemistry that governs sugar behavior, with implications across biochemistry, pharmaceuticals, and biotechnology.
The Classical View: Carbon 1 as the Anomeric Carbon
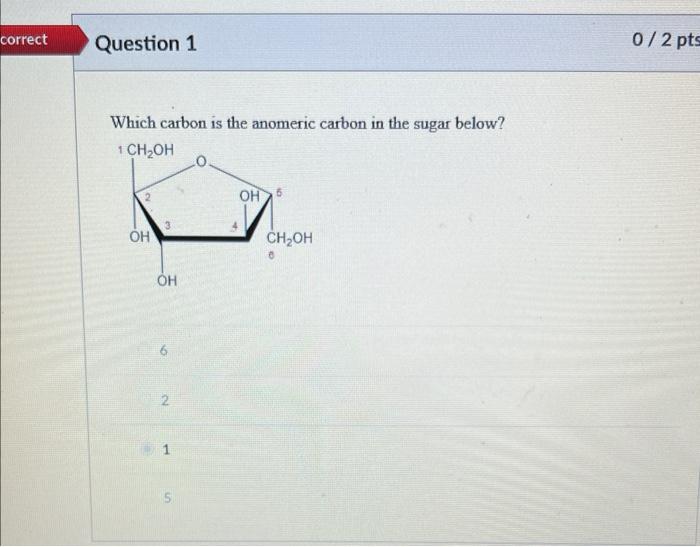
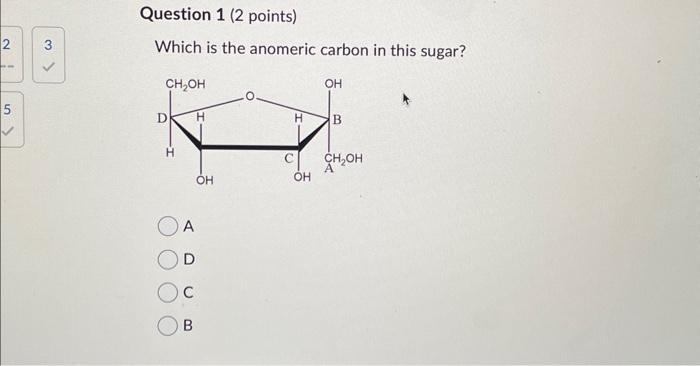
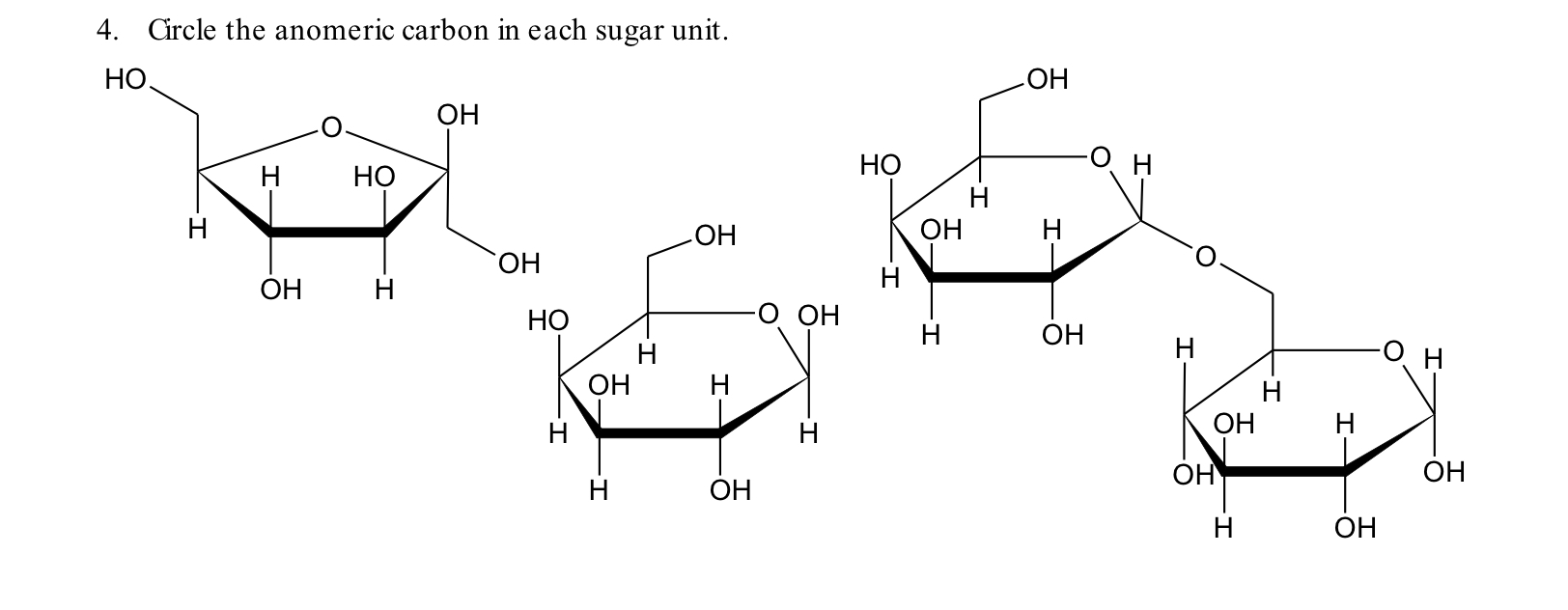
In standard carbohydrate chemistry education, the anomeric carbon is consistently defined as carbon 1 — the chiral center formed when the open-chain aldehyde or ketone functional group cyclizes via hemiacetal or hemiketal formation. This carbon is central to the distinction between alpha and beta anomers, labels that describe the stereochemistry of the hydroxyl group attached to the anomeric carbon relative to the ring oxygen. The anomeric effect, a key stabilizing influence in sugar conform

Related Post

A Remarkable Influence: Kennisandra Jeffries Shapes a Generation Through Advocacy and Leadership for Kids
Exploring the Footprint of Allisson Lozz Tv Shows

Nepal U20 vs Palestine U20: A Matchup That Shook the U-20 World Cup Stage

Brunswick: Unpacking Its Mystery, Origins, and Global Influence

