Ionic Compound vs Molecular Compound: Unlocking the Key to Chemical Structure
Ionic Compound vs Molecular Compound: Unlocking the Key to Chemical Structure
At the heart of modern chemistry lies a distinction that defines how atoms bond and form the building blocks of matter: the difference between ionic and molecular compounds. While both arise from electron interactions, their underlying structures, formation mechanisms, and physical properties diverge fundamentally—carving distinct roles in industries ranging from pharmaceuticals to materials science. Understanding when and why one forms over the other reveals not just chemical principles, but the real-world implications of molecular architecture.
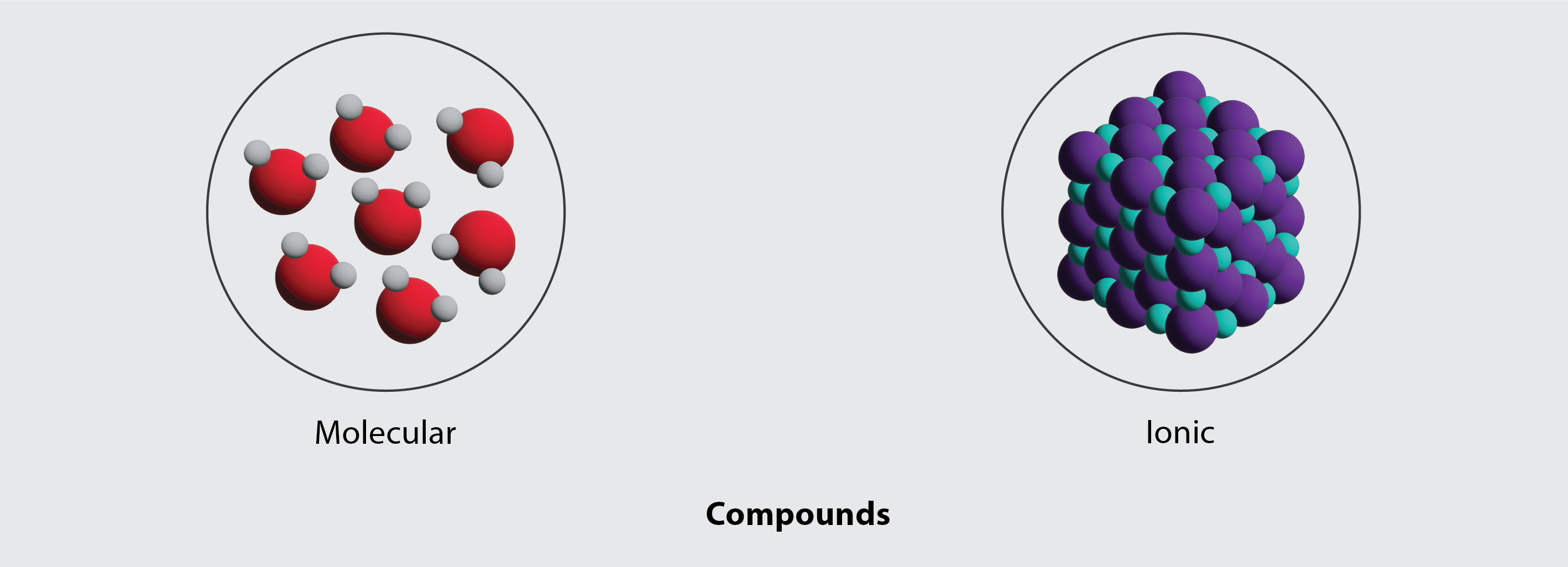
Understanding Bonding Kinetics Ionic compounds emerge when atoms transfer electrons rather than share them, a process driven by large electronegativity gaps—typically between metals and nonmetals. Metals, eager to shed electrons, release positive ions (cations), while electronegative nonmetals gain electrons to form negative ions (anions). The electrostatic attraction between these oppositely charged ions creates a rigid, crystalline lattice structure.
This bond is non-directional, relying instead on long-range Coulomb forces—what physicist Richard Feynman once described as “reducing chemistry to physics.” In contrast, molecular compounds form through **covalent bonding**, where atoms *share* electrons to complete outer shells. These bonds are directional and concentrated between specific atoms, resulting in discrete, finite molecules held together by stronger, yet shorter-range intermolecular forces such as hydrogen bonds or van der Waals attractions.
The Structural Divide: Lattices vs.
Molecules  The physical architecture of ionic and molecular compounds reflects their bonding nature. Ionic compounds assemble into repeating three-dimensional lattice networks that extend throughout the entire crystal. This arrangement results in high melting and boiling points—often exceeding 1,000°C—due to the immense energy required to overcome electrostatic forces.
The physical architecture of ionic and molecular compounds reflects their bonding nature. Ionic compounds assemble into repeating three-dimensional lattice networks that extend throughout the entire crystal. This arrangement results in high melting and boiling points—often exceeding 1,000°C—due to the immense energy required to overcome electrostatic forces.
Ionic solids also tend to be brittle, their rigid lattice cracking easily under stress. By contrast, molecular compounds form isolated molecular units with defined chemical identities. Their moderate melting points—often below 300°C—reflect weaker intermolecular attractions rather than strong intramolecular covalent bonds.
This structural disparity directly influences solubility: ionic compounds dissolve readily in polar solvents like water, where hydration shells stabilize individual ions, whereas molecular compounds often dissolve by disrupting solvent-solvent interactions, preserving molecular integrity. <>()
Case in point: sodium chloride (NaCl), a quintessential ionic compound, dissolves instantly in water to yield free Na⁺ and Cl⁻ ions that conduct electricity—a hallmark of ionic behavior. Conversely, sucrose (C₁₂H₂₂O₁₁), a molecular compound, remains suspended in water, remaining intact because hydrogen bonds between sucrose and water molecules preserve its bulk structure while coordinating dissolution.
<| category: Comparison | type: internal | id: icmp2025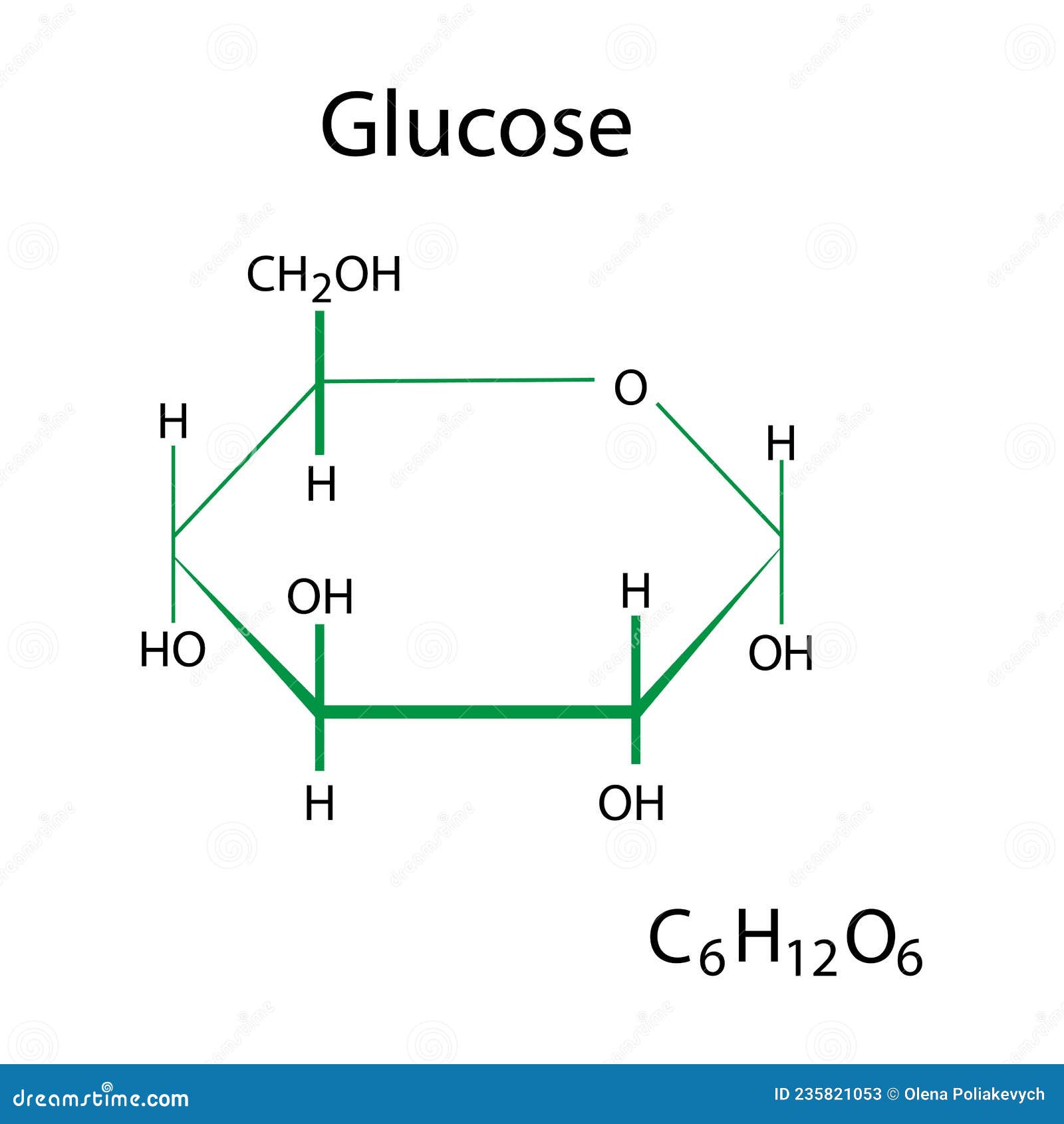

Related Post

The Krays’ London Underworld: The Infamous Twins Who Blazed Through Crime’s Shadows

Guam’s Titles of Triumph: Exploring the Legendary Ingang and the Majestic War in the Pacific
Mitsy270 Age Wiki Net worth Bio Height Boyfriend
Examining the Public Profile of Kash Patel’s Offspring: The Kash Patel Daughter Narrative

