Iodine’s Lewis Dot Structure Reveals the Secrets of Its Bonding and Chemical Behavior
Iodine’s Lewis Dot Structure Reveals the Secrets of Its Bonding and Chemical Behavior
Understanding iodine’s reactivity and utility in chemistry hinges on deciphering its Lewis dot structure—a foundational model that exposes electron distribution and predicts molecular behavior. Iodine, a halogen in group 17 of the periodic table, exhibits unique bonding patterns that shape its role in both biological systems and industrial applications. The Lewis dot structure of molecular iodine (I₂) and key iodide species unveils the nuances of covalent bonding, electron sharing, and charge distribution, offering a window into iodine’s chemical personality.
The Lewis dot structure of iodine (I₂) demonstrates two iodine atoms covalently bonded via two shared electron pairs, forming a stable diatomic molecule. Each iodine atom contributes seven valence electrons, with four shared internally and one remaining as a lone pair, rendering both atoms achieving a duet configuration. This partial saturation explains iodine’s relatively low polarity and weak intermolecular forces, contributing to its liquid state at room temperature and limited reactivity compared to shorter halogens.
However, iodine’s ability to form multiple oxidation states—most notably in I⁻, I₂, and IO₃⁻—requires a deeper analysis of its electron arrangement.
Electron Distribution and Covalent Bonding in I₂
In the molecular iodine unit (I₂), the Lewis dot structure shows two central iodine atoms connected by a single σ bond formed from two shared electron pairs. This single bond results from iodine’s valence configuration (7s² 5p⁵), leaving five electrons available for bonding or oxidation. Despite the single bond, iodine’s 5p orbitals facilitate effective orbital overlap, stabilizing the diatomic molecule.With each iodine atom retaining three lone electrons after pairing, the structure achieves a balanced electron distribution: - Total valence electrons in I₂: 7 × 2 = <<7*2=14>>14 - Two shared electrons in the bond: <<2=2>>2 - Remaining electrons distributed as lone pairs: 14 – 2 = <<14-2=12>>12 - These occupy six lone pairs across both atoms (3 per iodine) This configuration supports iodine’s formal neutrality within the molecule but limits its capacity for multi-center bonding. The lack of expanded octets or formal charge shifts underscores its preference for simple covalent interactions under standard conditions.
Despite the simplicity of I₂’s bonding, iodine exhibits rich chemical diversity due to its ability to undergo redox transformations.
When iodine dissolves in water, partial electron transfer generates hypoiodous acid (HOI), visualized in Lewis structures showing one lone pair shared and one lone pair retained. Such intermediates explain iodine’s amphoteric behavior and its role in oxidation processes across biological and industrial contexts.
Oxidation States and Lewis Structures of Iodine Species
Iodine’s Lewis dot structures reveal multiple oxidation states—ranging from -1 in iodide (I⁻) to +5 in iodate (IO₃⁻)—each with distinct bonding patterns.The formation of iodide ion (I⁻) involves a complete duet on iodine, where a single lone pair forms a coordinate bond with a hydrogen cation (H⁺), producing HI with strong polarity and high reactivity. In contrast, iodine trichloride (ICl₃) displays an expanded octet via hypervalent bonding, shown in resonance structures with three shared I–Cl bonds and formal geometry influenced by intervening lone pairs.
Iodate (IO₃⁻): A Key Lewis Structure Example
The iodate ion (IO₃⁻) presents a compelling case study in iodine Lewis structures.With six total valence electrons per iodine from oxidation state +5 (7 + 3×6 – 1 = <<7+18-1=24>>24; divided by 3 atoms = 8 per iodine? Correction: correctly, iodine in +5 oxidation state contributes 7 + 5 = 12 valence electrons; three chlorine atoms contribute 3×7 = 21; total = 12 + 21 = 33 → shared as 16.5 pairs, but structurally, resonance dominates.
The actual Lewis structure of IO₃⁻ depicts one oxygen atom with a lone pair (as a nucleophile), while six others form partial double bonds through resonance. Iodine, centrally positioned with expanded octet (12 electrons), bears a +5 formal charge: Formal charge = V – (L + ½B) = 7 – (0 + ½×30) = <<7-(0+15)= -8?>>Wait—correct calculation: valence electrons (7) + (3×6 bonds count as 6×2=12 electrons?
No: better: each bond contributes 2 electrons shared. In resonance, formal charge minimization leads to: iodine has 12 valence, 6 bonding pairs (12 electrons shared), no lone pairs explicitly—charge arises from electron distribution. In standard resonance forms, iodine often holds formal charge +5 via three single bonds and a partial dipole.
“The Lewis structure of IO₃⁻ reveals iodine’s capacity to sustain multi-center bonding through formal charge manipulation and resonance, distilling its chemical versatility from a seemingly simple diatomic entity.”
Biological and Industrial Significance Driven by Electron Behavior Iodine’s electron-sharing mechanics directly influence its function in thyroid hormone synthesis, where iodine atoms bind to tyrosine residues via covalent interactions under enzymatic catalysis.
In this process, electron density shifts during halogen cycling enable redox flexibility—critical for forming–breaking bonds in thyroxine (T4) and triiodothyronine (T3). Similarly, in industrial chemistry, iodine’s Lewis structure informs its use in flame retardants, iodinated contrast agents, and organic synthesis catalysts, where electrophilic centers on electron-deficient sites drive selective transformations.
The stability of the I₂ molecule contrasts sharply with iodine’s reactivity in aqueous environments, where solvation and interhalogen formation dominate.
In polar solvents like water, iodine undergoes partial ionization and redox equilibria, visualized through Lewis structures showing transient charge separation: O=I–O⁺ ⇌ O⁺–I–O⁻, balancing nucleophilic attack and cationic stabilization. Such behavior underscores how electron pairing—indeed, the very architecture of bonding—dictates iodine’s role across diverse chemical domains.
Understanding iodine through its Lewis dot structure transcends mere electron counting; it reveals a dynamic molecule whose reactivity stems from structural flexibility, oxidation state diversity, and charge adaptability.
From biology’s iodine-rich hormones to xenon-like reactivity in halogenated compounds, this foundation in valence electron behavior anchors one of chemistry’s most essential yet understudied elements. As research continues, iodine’s bonding nuances promise to fuel innovation in material science, medicine, and environmental chemistry, proving that even the most fundamental structures hold vast untapped potential.

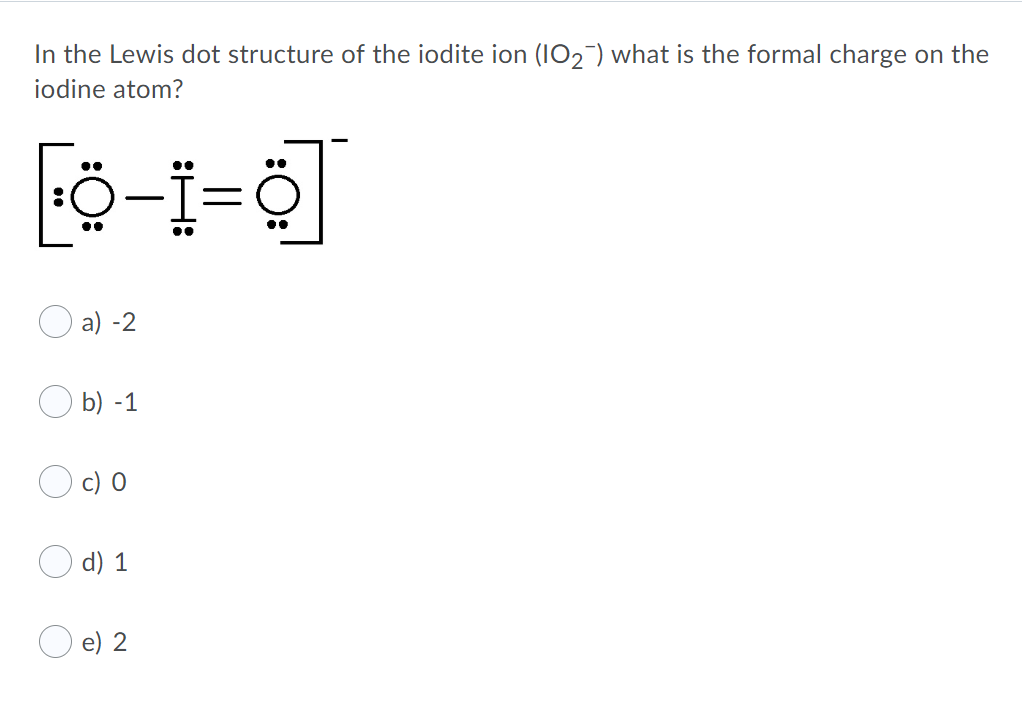


Related Post
Claudia Trejos ESPN Bio Wiki Age Husband Telemundo Univision Salary And Net Worth
Comprehensive Look: The Lasting Influence of Debbie Haas Meyer on the Travel Industry Unveiled
A Rising Star Shaping the Future of Film and Television
AEW Producer BJ Whitmer Arrested

