Inside the Tubular Gateway: How the Fallopian Tubes Safeguard Ovum Production and Fertility
Inside the Tubular Gateway: How the Fallopian Tubes Safeguard Ovum Production and Fertility
The fallopian tubes—often overshadowed by the ovaries and uterus—play an indispensable yet underappreciated role in women’s reproductive health. As a critical link in the female reproductive system, the fallopian tubes serve not only as conduits for ovum transport but also as dynamic environments where early fertilization occurs and hormonal signals fine-tune ovulation. Central to their function is the fallopian tube’s complex inner lining, particularly the fallopian tube epithelium, where structures resembling the "tubal fallop Open the passage to a detailed exploration of how the ovidukal (fallopian) environment supports ovum development, maturation, and eventual release.
This article reveals how the fallopian tubes, especially the fallopian fallop—referring to the fallopian epithelium and its functional microenvironment—operate as a vital, active component in reproducing human life, ensuring that ovum production reaches full biological potential.
The Ovidukal Function: More Than Just a Passageway
Contrary to the common perception of fallopian tubes as passive channels, modern reproductive biology underscores their active role in nurturing and guiding ovum production. The epithelium lining the fallopian tubes—often called the fallopian tube epithelium or fallop—possesses specialized ciliated and secretory cells that orchestrate the journey of the ovum from ovulation to potential fertilization.These cells release nutrients, ions, and signaling molecules that regulate fluid balance and create a receptive microclimate essential for ovum viability. “Far from being just a conduit,” explains Dr. Amina Diallo, reproductive endocrinologist at the Institut Fondamental de Recherche Médicale, “the fallopian tubes actively filter and support each ovum, optimizing conditions for fertilization and early embryo development.” This dynamic environment involves precise coordination with ovarian hormones such as estrogen and progesterone, ensuring ovum maturation aligns with systemic reproductive cycles.
Visually, the fallop-ud structures change along the tube’s length: from the narrow infundibulum (near the ovaries) to the broad ampulla, where fertilization most frequently occurs. The ampulla’s expansive lumen and rich vascularity create ideal conditions, supported by a layered epithelium that transitions from pseudostratified ciliated columnar to non-ciliated simple columnar cells—each layer contributing to ovum conditioning.
This specialized environment not only preserves the ovum’s structural integrity but also protects it from external infection or premature degradation.
The fallop’s mucosal surface continuously secretes a nutrient-rich fluid, rich in glycoproteins, bicarbonate ions, and growth factors—components critical for sperm capacitation and early embryonic growth. Disruption in this finely tuned system, whether due to inflammation, scarring, or structural abnormalities, can compromise ovum transport and reduce fertility. Thus, fallop—synonymous with the functional epithelium and its complex biology—emerges as a cornerstone in reproductive physiology.
The Cellular Architecture: Cilia, Transport, and Fluid Dynamics
The fallopian tube epithelium functions as a coordinated biological machine, powered by personalized cellular components.Ciliated cells leap into action, rhythmically beating to propel the ovum toward the uterus. This coordinated ciliation ensures forward motion, preventing backflow and guiding ovum progression with remarkable precision. Supporting this movement are peg cells and secretory cells, which maintain fluid homeostasis and contribute to the tubal microenvironment.
“In every cycle, the epithelial layer adapts to hormonal cues,” notes Dr. Diallo, “increasing ciliary beat frequency at ovulation to enhance ovum transport efficiency.” This adaptability reflects the fallop’s role as a responsive, hormonally-driven system rather than a static structure. Malfunction—such as ciliary immotility or disrupted ion transport—can severely impair ovum movement and increase risk of ectopic implantation or infertility.
Beyond motility, the tubal epithelium monitors ovum quality through tight junctions and receptor signaling. These mechanisms allow selective interaction with sperm, ensuring only viable ovum candidates are targeted for fertilization. This cellular precision underscores why fallop health directly correlates with reproductive outcomes.
Clinical Relevance: Fallop Pathology and Fertility Outcomes
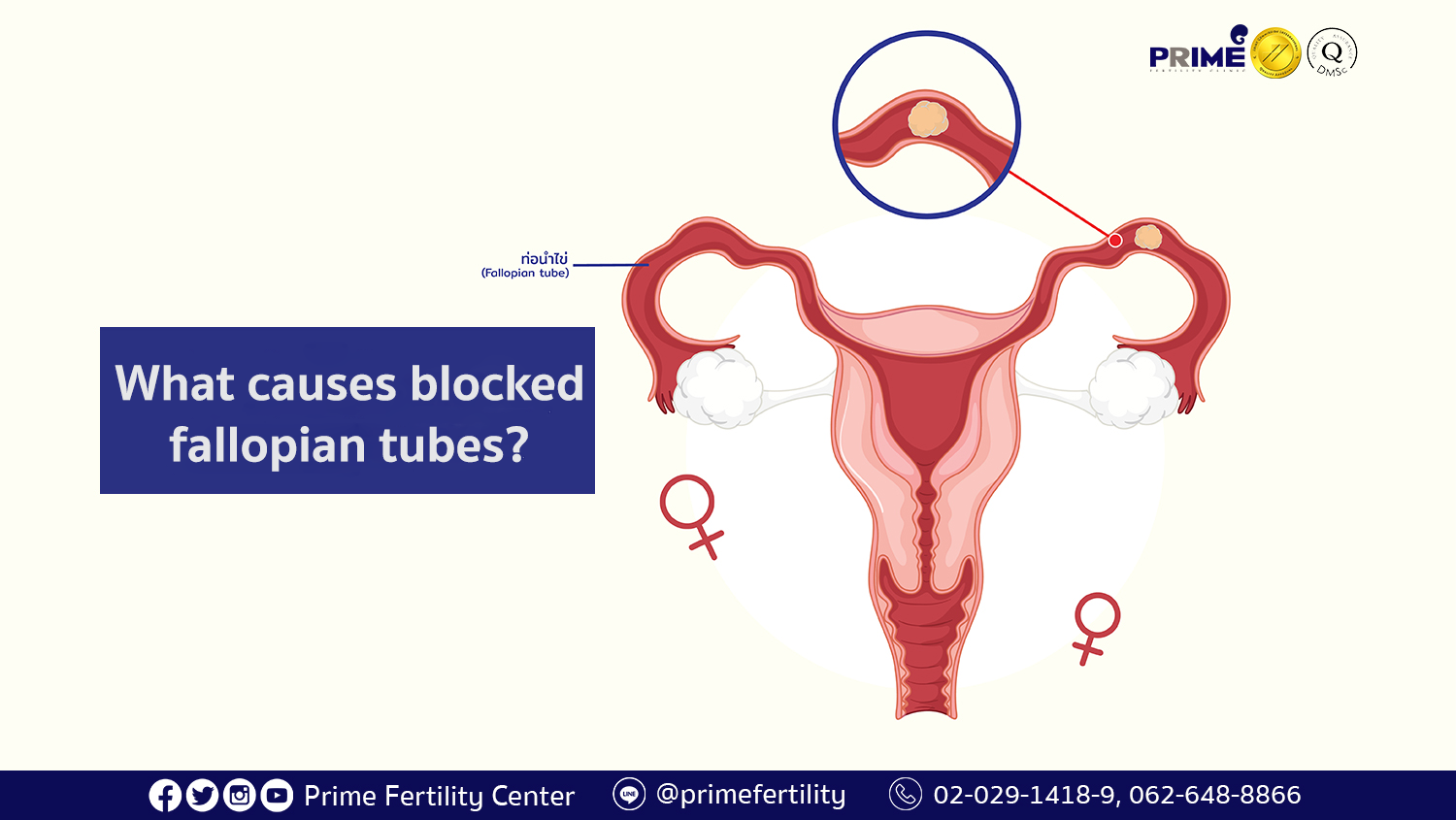
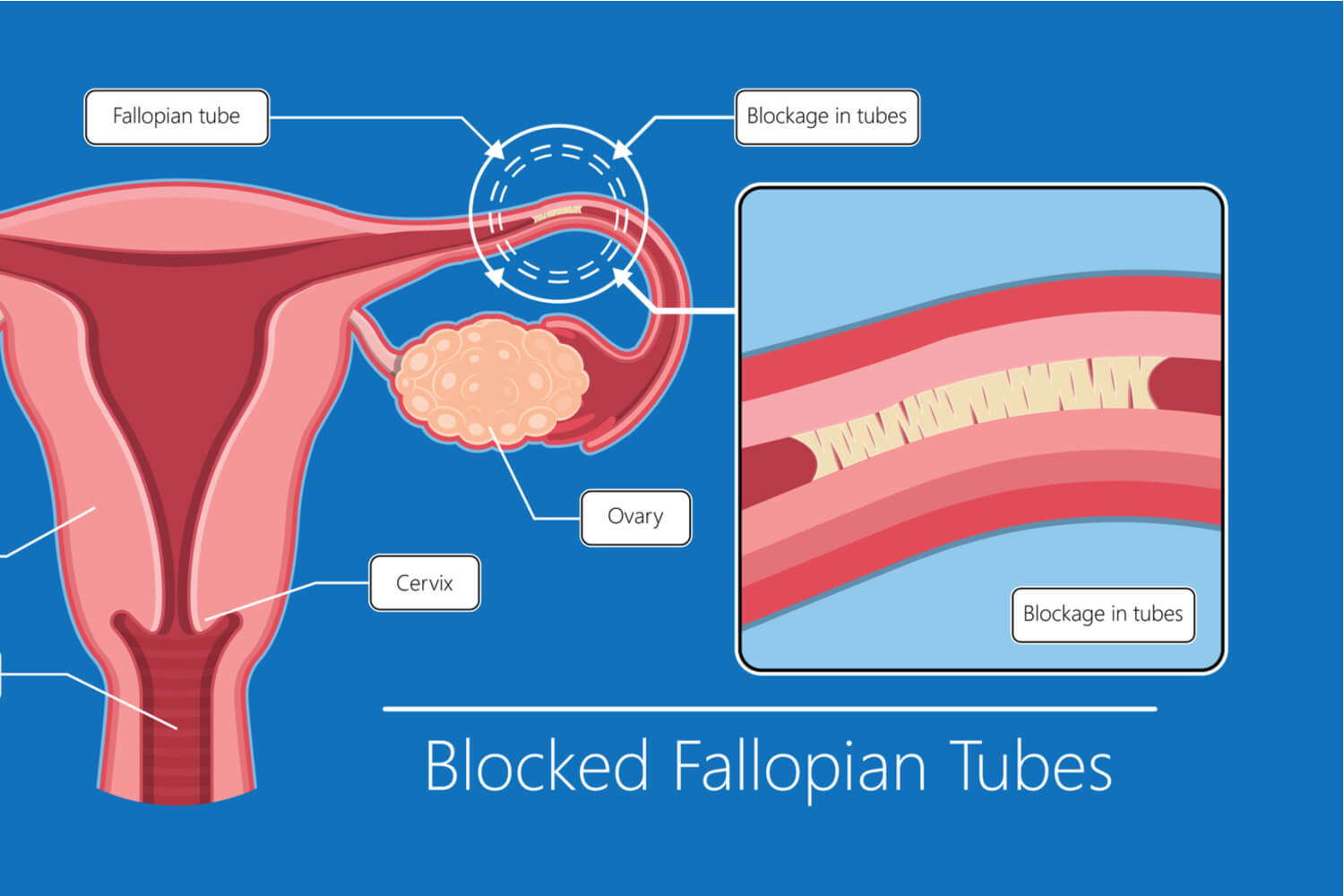
Structural and functional abnormalities in the fallopian tubes profoundly influence women’s fertility.Common disorders include salpingitis (infection-induced inflammation), tubal adhesions from prior infections like chlamydia, and congenital malformations. Each condition disrupts fallop function, often leading to obstruction, reduced ciliary activity, or impaired fluid secretion. “When fallop function deteriorates,” explains Dr.
Diallo, “the ovum may remain in transit too long, increasing degradation risk or failing to reach the ampulla in time for fertilization.” Such disruptions are a leading cause of tubal infertility, affecting millions globally. Diagnostic imaging such as hysterosalpingography and modern laparoscopic evaluations allow precise detection of tubal damage. Innovative treatments aim to preserve or restore fallop function.
Techniques like microsurgical salpingectomy, tubal reanastomosis, and emerging regenerative medicine approaches—including stem cell therapies targeting epithelial repair—offer hope. Additionally, assisted reproductive technologies such as IVF bypass fallopian dysfunction entirely, yet understanding fallop biology remains vital for optimizing intrauterine embryo transfer timing and enhancing implantation success.
Emerging Frontiers: Research and Future Directions
Ongoing research into the fallopian fallop epithelium is transforming reproductive medicine.Advances in live imaging, single-cell transcriptomics, and organoid culture systems enable scientists to map cellular dynamics and molecular signaling with unprecedented detail. Studies reveal previously unknown populations of stem-like cells within the tubal lining, suggesting potential for in vivo regeneration. “Understanding how fallop actively constructs and maintains ovum microenvironments opens doors to novel therapeutics,” states Dr.
Amina Diallo, “especially for patients with hidden tubal pathologies where standard assessments miss subtle dysfunction.” Future prospects include targeted drug delivery systems that restore epithelial secretion, bioengineered scaffolds for tubal reconstruction, and personalized diagnostic panels predicting fallop health before fertility treatment. These innovations promise to elevate reproductive care, ensuring fewer women face infertility due to undetected fallop compromise.
Recognizing the fallopian tubes—especially the functional fallop—as a dynamic reproductive hub elevates their status from overlooked anatomy to central player in human reproduction.
The fallopian fallop epithelium is far more than a passive tube lining; it is a sophisticated biological network that collects, nurtures, transports, and protectively shelters the ovum.Its intricate interplay of cilia, secretory cells, and hormonal responses ensures that each ovum reaches its full potential in the race toward fertilization. Disruption at this stage reverberates through fertility, making fallop health a critical determinant in reproductive success. As scientific insight advances, so too does the potential to safeguard and enhance this essential system—affirming that in the symphony of human reproduction, the fallopian tubes play a starring role.

Related Post
Zodwa Wabantu Biography Real Age and How She Got into Dancing

Bill Paxton’s First Marriage Revealed: A Foundational Chapter in His Public Life

Tobias Menzies Relationships: Unpacking the Man Behind the Characters

