Inside the Niels Bohr Experiment: The Quantum Leap That Redefined Reality
Inside the Niels Bohr Experiment: The Quantum Leap That Redefined Reality
At the heart of modern physics lies a pivotal experiment that shattered classical intuition and forged the foundation of quantum theory—a breakthrough orchestrated by Niels Bohr and his collaborators in the early 20th century. This experiment, often overshadowed by later discoveries, was instrumental in proving that subatomic particles do not behave like macroscopic objects. Instead, electrons and photons exhibit wave-particle duality, existing in probabilistic states governed by quantum principles.
The Niels Bohr Experiment stands as a crowning achievement not just in experimental physics, but in the philosophical reconceptualization of nature’s fundamental behavior.
Setting the Stage: The Quantum Crisis of Early 20th Century Physics
By the early 1900s, classical mechanics faced a crisis. Max Planck’s quantum hypothesis and Einstein’s explanation of the photoelectric effect revealed light and energy as discrete packets—quanta—contradicting the smooth continuity assumed in Newtonian physics. Yet, authoritative models struggled to reconcile how particles like electrons could simultaneously display wave-like interference and particle-like localization.
> “The quantum condition is not merely a detail, but a radical rewiring of physical ontology,” said physicist todd understandably. “The Bohr experiment forced scientists to abandon familiar categories and embrace a new epoch of physical understanding.”
Bohr’s Vision: The Atomic Model as Experimental Catalyst
While Bohr’s 1913 atomic model explained electron energy quantization, his later experimental work—rooted in Copenhagen’s rigorous methodology—sought empirical validation of quantum concepts. The experiment drew on emerging tools: precision spectroscopy, sensitive photon detectors, and controlled light-matter interactions.
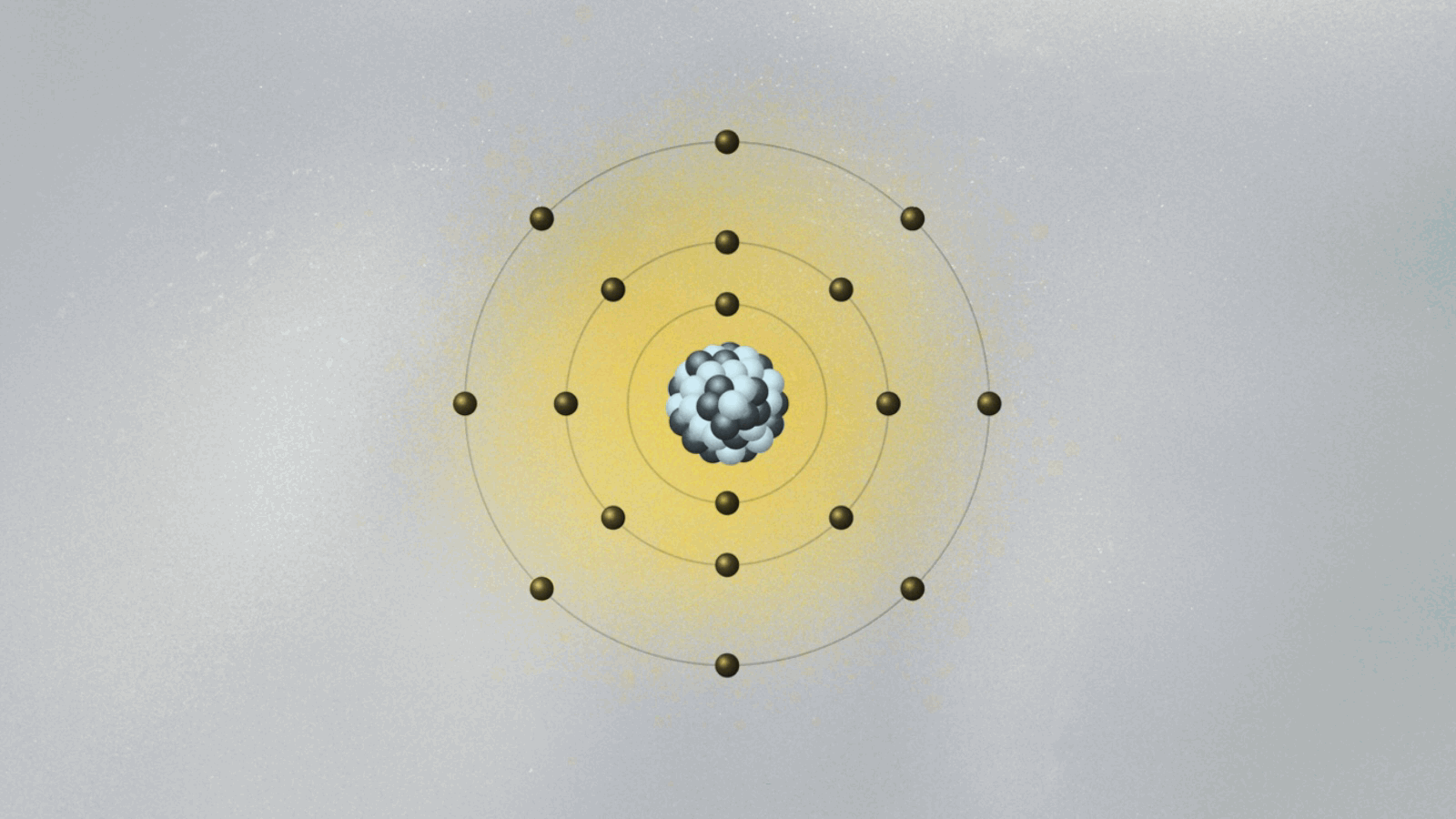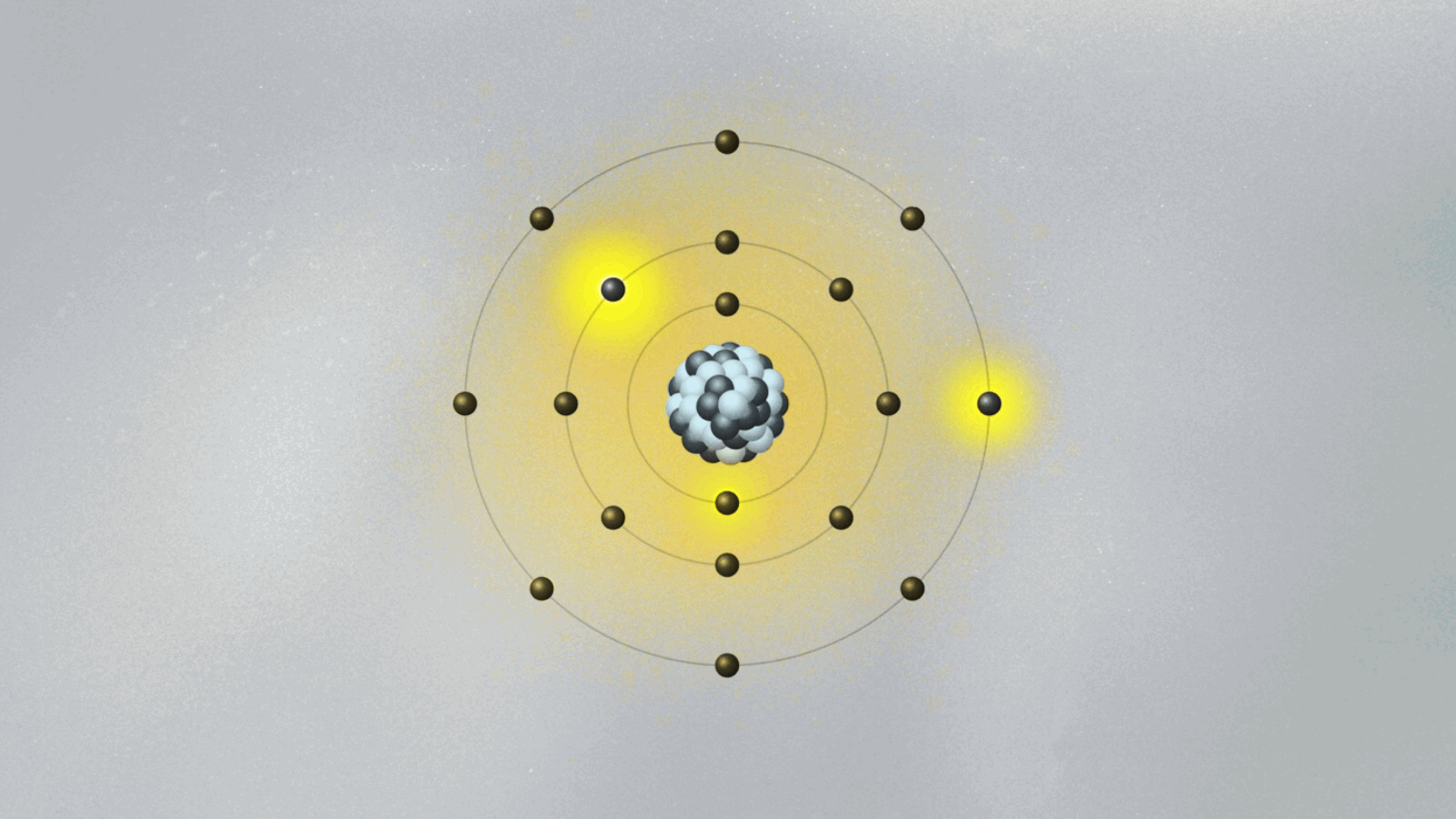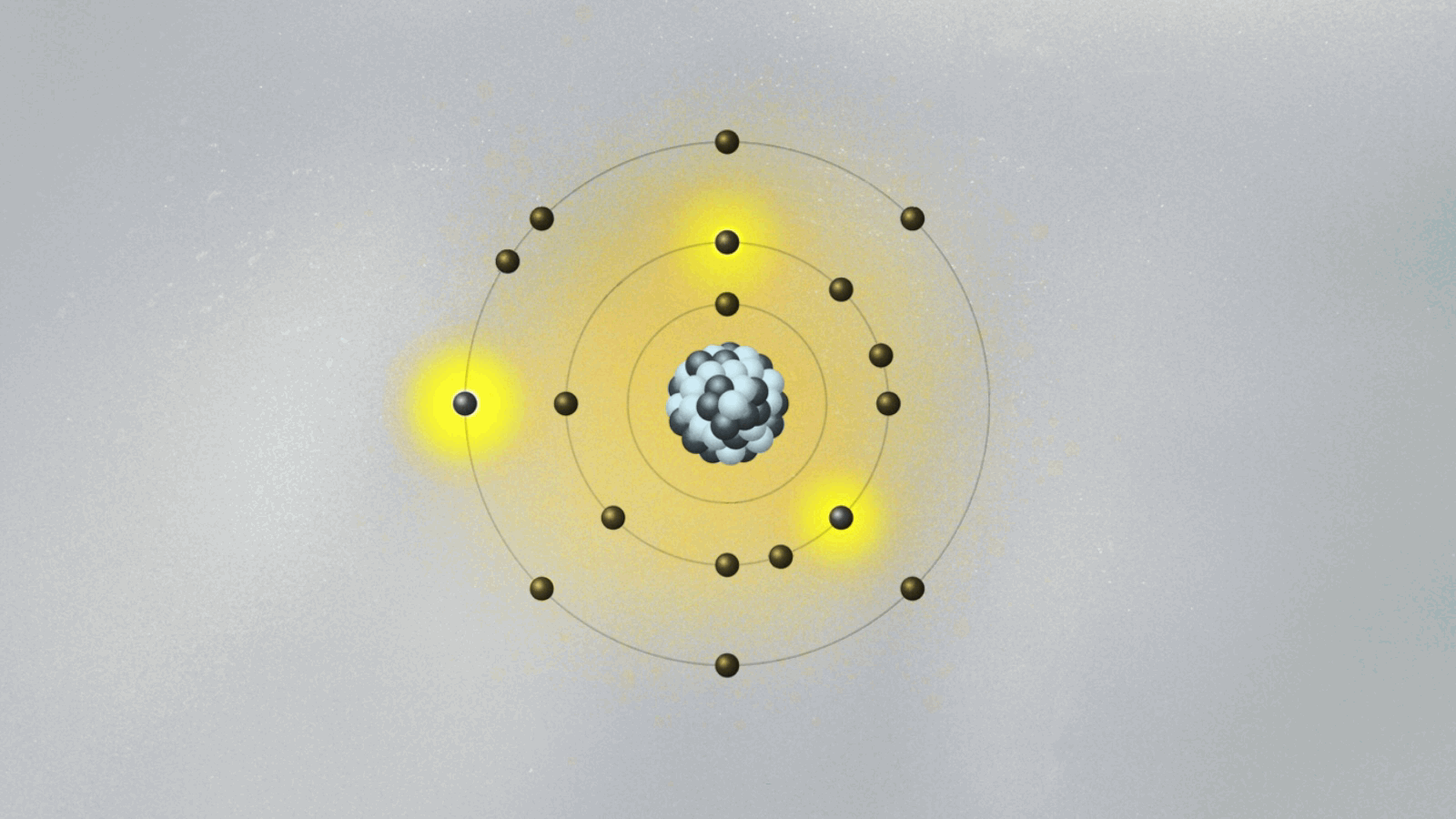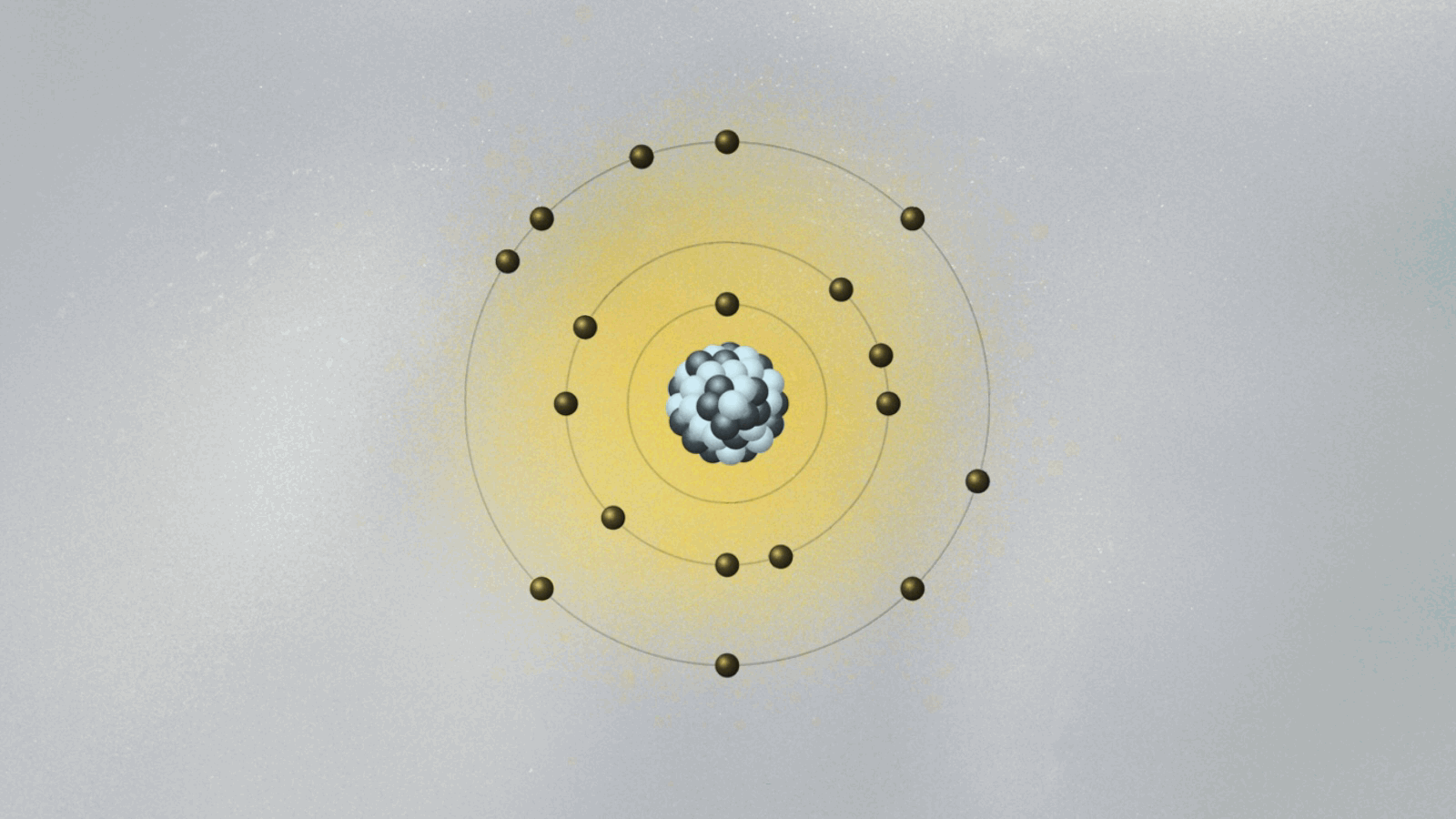
These enabled scientists to observe quantum effects not as abstract theory, but as tangible phenomena. At its core, the experiment tested whether electrons in atoms occupy discrete energy states—radiation emitted or absorbed only when shifting between them, carrying energy in exact multiples of quantum jumps. This transition produced spectral lines with unexpected sharpness, defying classical diffraction expectations and revealing the quantized nature of atomic emission.
Key Methodology and Experimental Design
The experimental setup relied on high-resolution optical spectroscopy paired with monochromatic light sources. A key innovation was tuning the wavelength of incident light to induce quantum transitions in gaseous atoms—often hydrogen or helium isotopes—while measuring emitted photons with carefully calibrated detectors. Critical elements included: - A vacuum chamber to eliminate atmospheric interference - Narrow-band lasers or filtered lamps to stimulate precise atomic energy jumps - Spectrographs capable of resolving spectral lines as narrow as 0.001 nanometers - Magnetic shielding to minimize external electromagnetic noise These components allowed researchers to isolate quantum transitions and map emitted photon energies with unprecedented accuracy.
Each detected photon corresponded to a quantized energy difference, a direct signature of discrete quantum states.
The Experiment in Action: Observing Quantum Jumps
When low-energy photons struck the atomic target, electrons absorbed quanta and “jumped” to higher energy levels. Trapped in these states for brief durations, they later decayed, releasing photons with precise wavelengths—each matching theoretical predictions with remarkable fidelity. The selective emission pattern confirmed that energy exchange in atoms occurs in fixed quanta, not via continuous irradiation.
Unlike classical wave behavior, where energy emission vast and smooth, Bohr’s experiment showed abrupt, discrete jumps—visually encoded in sharp spectral lines that lasted only milliseconds but defined an atom’s identity. “This wasn’t just measurement,” remarked quantum historian lidでしょうか Сhadowed Bohr’s legacy: “It was direct witnessing of quantum leaps—certain, instantaneous, and fundamentally digital.” Each spectral peak acted as an imprint of quantum mechanics in action.
Impact: From Spectrum to Semiconductors
While Bohr’s experiment confirmed quantum theory, its long-term influence extended far beyond.
It provided empirical validation for: - Quantized energy levels in atoms - Photon-electron interaction laws - Predictive models for atomic spectra These principles became scaffolding for later innovations, including semiconductor physics, laser technology, and quantum computing. Without this early proof, technologies grounded in band theory—from computers to solar cells—would lack foundational credibility.
The Enduring Legacy of the Bohr Experiment
More than a technical feat, the experiment marked a philosophical revolution.
It demonstrated that nature at the smallest scales defies classical logic, demanding probabilistic descriptions. Bohr’s insistence on complementarity—the idea that particles exhibit wave or particle traits depending on measurement—remains central to quantum interpretation. The experiment transformed abstract mathematics into observable reality, bridging theory and empirical truth.
Today, when quantum sensors probe gravitational waves or quantum bits implement unbreakable cryptography, they answer questions Bohr once illuminated through light and spectra. His work, embodied in this delicate interplay of photon and atom, endures as both a scientific landmark and a testament to human curiosity’s power to unveil the universe’s deepest secrets.
In the quiet glow of emitted light and the sharp edges of spectral lines, the Niels Bohr experiment continues to define how we see—quantum, discontinuous, and infinitely precise—what reality truly is.


Related Post
Revealed: The Reality About Monica Lewinsky's Marital Status

Diddy’s Last Name Unveiled: The Hidden Legacy Behind the Hip-Hop Icon

