HowToTurnMolesIntoGrams: The Essential chemistry-to-gram conversion guide
HowToTurnMolesIntoGrams: The Essential chemistry-to-gram conversion guide
In chemistry, precise measurements dictate success—whether in a laboratory, classroom, or industrial setting. Converting moles to grams is foundational to chemical calculations, enabling accurate dosing, reaction planning, and quality control. Understanding how to transform between these units bridges abstract theory and real-world application, turning moles—a concept rooted in atomic counting—into grams that guide practical work.
This guide demystifies the process, ensuring clarity and reliability for scientists, students, and professionals alike.
The Molecular-Mass Bridge: Moles and Grams Explained
At the core of chemical quantification lies the mole—a unit representing 6.022 × 10²³ (Avogadro’s number) particles of a substance, whether atoms, molecules, or ions. Grams, by contrast, measure mass, a physical property tied to atomic weight. To convert moles to grams, molecules rely on molar mass, defined as the mass (in grams per mole) of one mole of a substance, dictated by its elemental composition.
For instance, hydrogen (H₂) has a molar mass of 2.016 g/mol—calculated from 2 atomic masses of hydrogen (≈1.008 g/mol each).
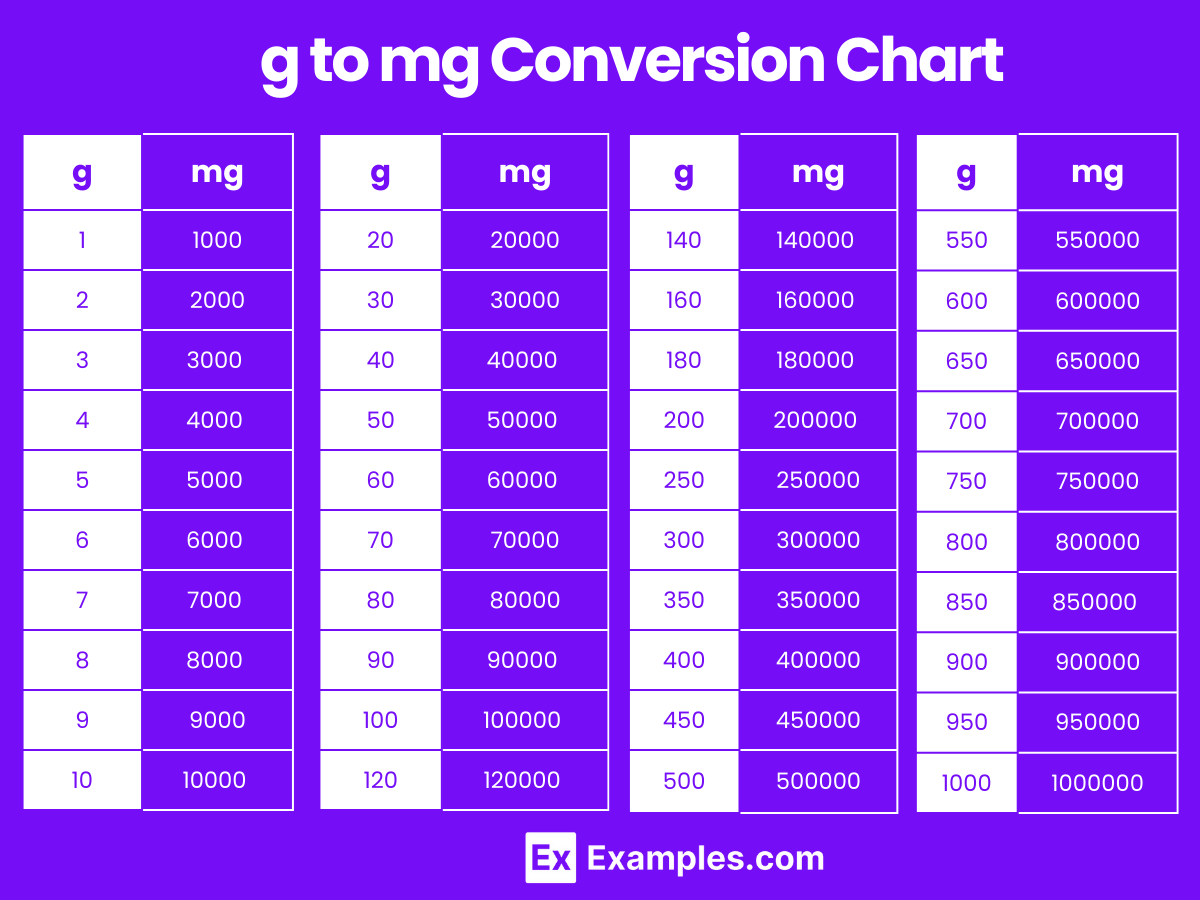
This simple relationship forms the backbone of the conversion: Grams = Moles × Molar Mass
—a formula that, when mastered, unlocks precision in countless chemical endeavors.Step-by-Step: Turning Moles into Grams like a Pro
Translating moles into grams follows a straightforward mathematical pathway, but precision in each step ensures accuracy. Below is a detailed breakdown of the process, validated by standard best practices in chemical education and industrial protocols.
- Start with the number of moles: Identify the amount of substance in moles (mol), whether given directly or derived from mass and molar mass.
- Determine the molar mass: Calculate the molar mass by summing atomic masses from the periodic table. Use standard isotopic averages (e.g., C = 12.01 g/mol, O = 16.00 g/mol).
- Apply the conversion formula: Multiply moles by molar mass.
The result is the mass in grams with correct significant figures, consistent with the least precise input value.
- Verify units: Ensure the final result expresses mass in grams (g), confirming dimensional consistency.
For example, converting 0.5 moles of water (H₂O), with a molar mass of 18.015 g/mol, yields: 0.5 mol × 18.015 g/mol = 9.0075 g, rounded appropriately based on significant figures.
Precision matters. Always preserve significant figures aligned with the input data—rounding too early may compromise accuracy in subsequent calculations.Why Accuracy in Mole-to-Gram Conversion Matters
In fields ranging from pharmacology to materials science, even fractional gram discrepancies can skew experimental outcomes, dosing precision, or production quality. In drug development, for instance, precise molar quantities ensure consistent therapeutic effects, while in chemical manufacturing, gram-level accuracy prevents hazardous imbalances or waste.
Common pitfalls include:
- Miscalculating molar mass by omitting isotopic details
- Rounding prematurely, amplifying rounding errors in multi-step processes
- Mixing units—such as substituting grams for kilograms or grams for milligrams without conversion
Practical Tips for Error-Free Conversions
To ensure reliable results, follow these key practices:
- Use authoritative molar masses: Rely on certified values from the CRC Handbook or reputable chemical databases, not rough estimates.
- Flag significant figures explicitly: Round only at the final step, preserving all input precision until the output.
- Leverage dimensional analysis: Write conversion factors as ratios (e.g., 1 mol H₂O = 18.015 g / 1 mol) to minimize arithmetic errors.
- Validate with inverse checks: Estimate reverse conversion (grams ÷ molar mass ≈ moles) to confirm reasonableness.
Real-World Applications: From Labs to Industry
Converting moles to grams transcends textbook exercises, serving critical roles across science and engineering. In pharmaceutical research, chemists calculate active ingredient dosages by converting experimental molar masses to grams for tablet formulation. Industrial chemists scale reactions from gram-scale lab tests to ton-scale manufacturing, where mass precision prevents costly overuse or shortages.
Chemical synthesis also depends on accurate mass input: when designing a reaction pathway, engineers rely on precise mole-to-gram conversions to balance reactants, predict yields, and optimize reagent usage. Even routine tasks—like preparing solutions in education labs—demand gram-level accuracy derived from moles, reinforcing the conversion’s ubiquity.
Mastering this skill transforms theoretical chemistry into tangible, repeatable practice—essential for innovation and compliance.Mastering the Art: Tips and Common Mistakes
Even experts can err when rushing through conversions. Sticking to a disciplined approach minimizes risks:


Related Post

Courtney Cronin Husband: A Quiet Strength Behind a Life of Purpose

Kroger Parsons Avenue Columbus Ohio: A Pillar of Retail Innovation on a Bustling Urban Mainline

AWildSheepChase: Unraveling the Wild Dance of Survival and Spirit

Bride Style Carry: Perfecting Elegance, Poise, and Confidence in Every Step

