How Do You Find Molarity? The Precise Science Behind Concentration Calculations
How Do You Find Molarity? The Precise Science Behind Concentration Calculations
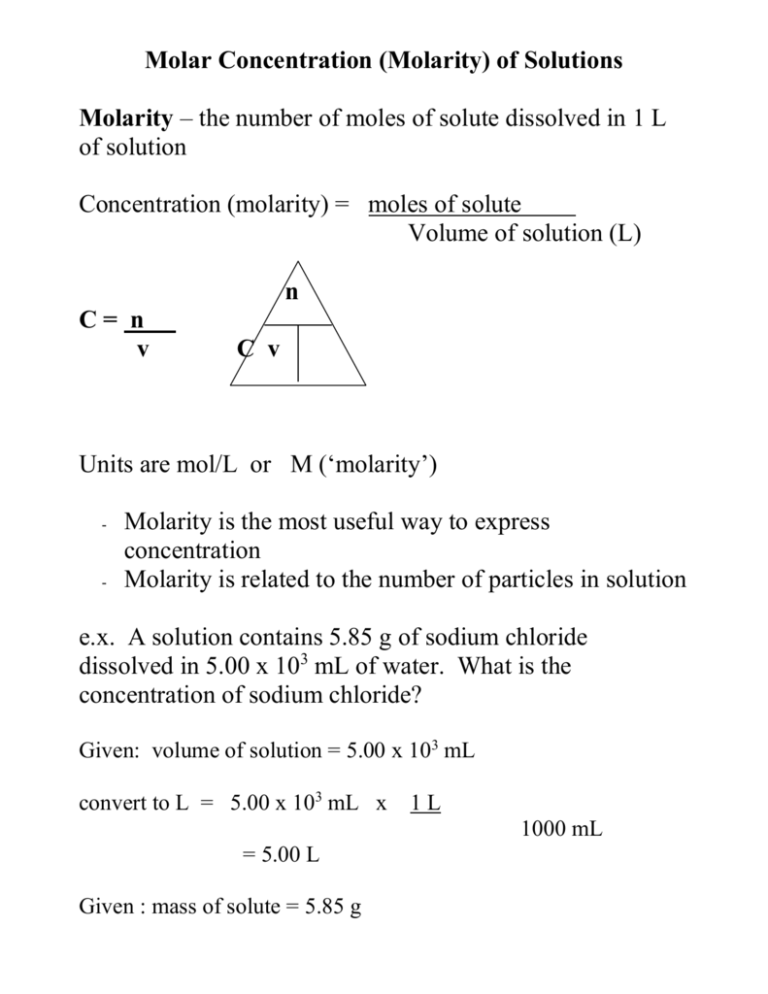
Finding molarity — the cornerstone of chemical concentration measurement — is not just a routine procedural task but a foundational skill in chemistry that underpins laboratories, pharmaceutical development, and environmental testing. Molarity, defined as moles of solute per liter of solution, directly influences reaction rates, equilibrium calculations, and stoichiometric precision. Yet, many practitioners still grapple with the exact methodology behind its determination.
This article demystifies the step-by-step process of calculating molarity, emphasizing accuracy, real-world relevance, and practical applications across scientific domains. Understanding molarity requires a clear grasp of its formula: \n}\forces{M\ (\text{molarity})}\ = \frac{\text{moles of solute}}{\text{liters of solution}}\\ where a mole is defined as 6.022 × 10²³ entities (Avogadro’s number) and volume is measured in liters. This definition anchors every calculation and ensures consistent, reproducible results across labs worldwide.
At the core of finding molarity lies a systematic sequence of steps that transforms raw data into actionable concentration values. Begin with determining the moles of solute — often the most critical and error-prone phase. If provided experimentally, such as in titration or gravimetric analysis, moles may already be known; otherwise, they must be derived from nominal mass and molar mass.
For a solute like sodium chloride (NaCl), whose molar mass is 58.44 g/mol, the conversion is straightforward: divide the measured mass (in grams) by the molar mass.
For instance, weighing 29.44 grams of NaCl yields \n}\fact{29.44\ \text{g} \div 58.44\ \text{g/mol} = 0.504\ \text{moles}}\\ \`. This step requires precision — even a 0.1-g deviation can shift molarity by approximately 1.7%. Especially in high-stakes applications like drug formulation or biochemical assays, digital balances with ±0.0001 g accuracy are essential to maintain reliability.
Next, volume of the final solution must be determined with exactness.
Unlike mass or density, solution volume depends on the precision of the container used — be it a volumetric flask, graduated cylinder, or beaker. A measured volume of 250.0 mL (0.250 L) is preferable to 250 mL, which lacks decimal clarity. Volumetric flasks, calibrated to maintain ±0.01 mL tolerance, are standard in analytical labs because they minimize systematic error.
University-level labs often enforce protocols requiring air-dried glassware and standardized measuring techniques to avoid parallax or meniscus misreads.
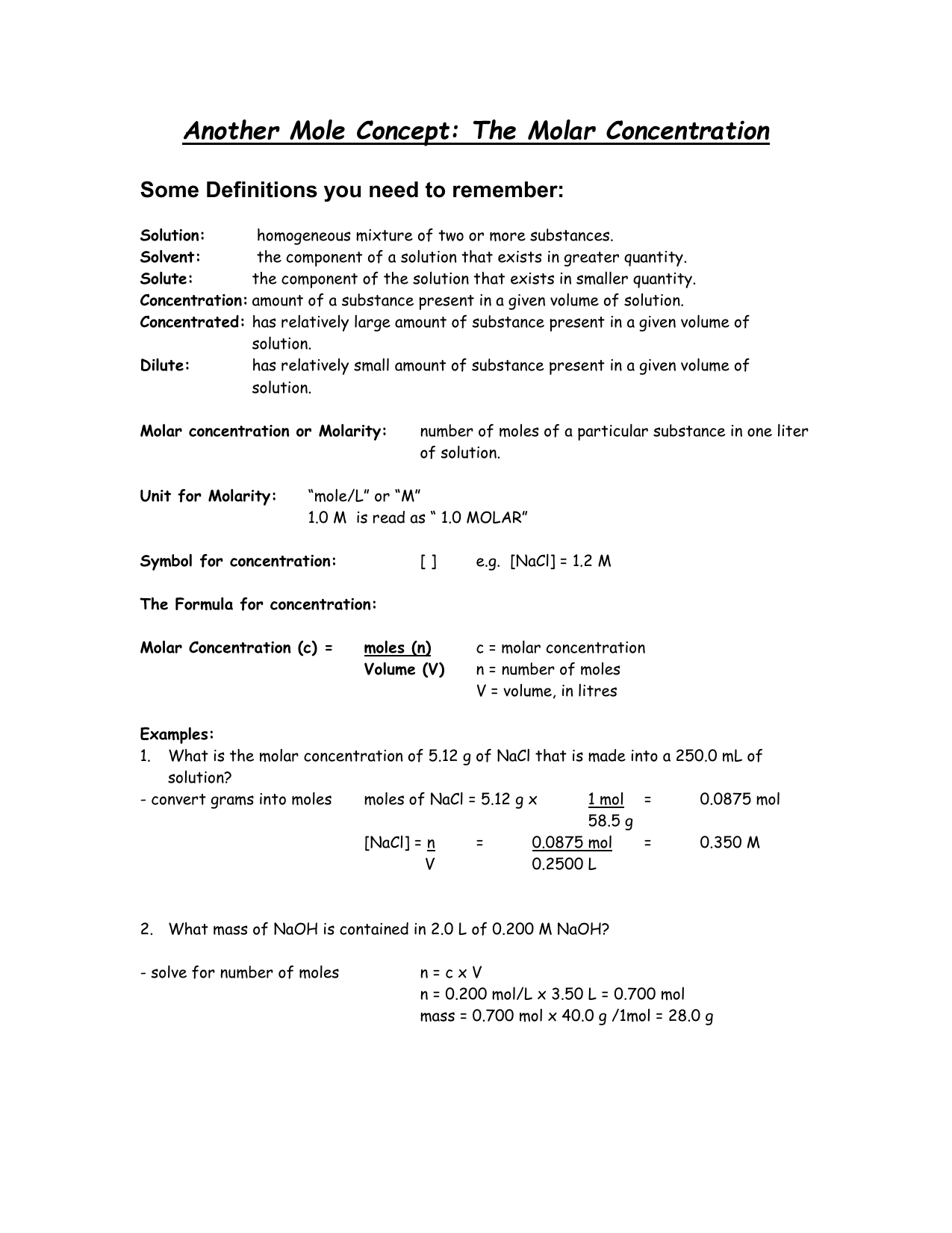
Example Calculation: From Data to Molarity
Suppose a student dissolves 5.85 grams of NaOH (molar mass = 40.00 g/mol) in water to prepare 250.0 mL of solution. \n1. Calculate moles of NaOH: \n}\fact{5.85\ \text{g} \div 40.00\ \text{g/mol} = 0.14625\ \text{mol}\\ \n2.Convert volume to liters: \n}\fact{250.0\ \text{mL} = 0.2500\ \text{L}\\ \n3. Compute molarity: \n}\fact{0.14625\ \text{mol} \div 0.2500\ \text{L} = 0.585\ \text{mol/L} \approx 0.585\ \text{M}\\
This result—0.585 M

Related Post

Supermarket Superstars: How Lyrics Transform Floral Merchandising in Modern Stores

Ready To Love’s Season 8 Shock: Beloved Cast Member Dies, Sparks Emotional Outpouring
Barron Trump Sings Unveiling the Untold Story: A Young Artist’s Journey Behind the Voice

