Fueling the World: Real-World Power of Endothermic and Exothermic Reactions
Fueling the World: Real-World Power of Endothermic and Exothermic Reactions
Every action from lighting a match to powering industrial engines hinges on a silent transformation occurring at the molecular level—chemical reactions that either absorb or release heat. These processes, classified as exothermic and endothermic, are fundamental to energy transfer in both natural and engineered systems. Understanding them reveals not only basic chemistry but also the principles behind technologies shaping modern life—from combustion engines to thermal energy storage.
From the instant warmth of a flame to the energy hidden in sugar molecules, these reactions underscore a world constantly in thermal flux.
The Science Behind Heat Changes: Defining Endo and Exo
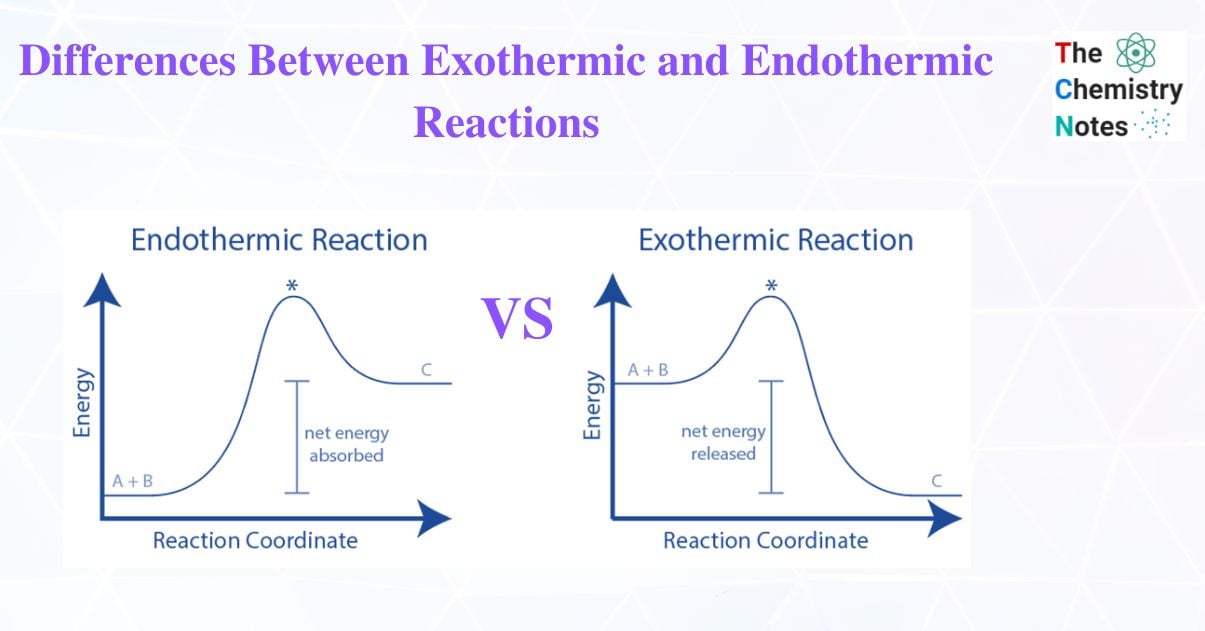
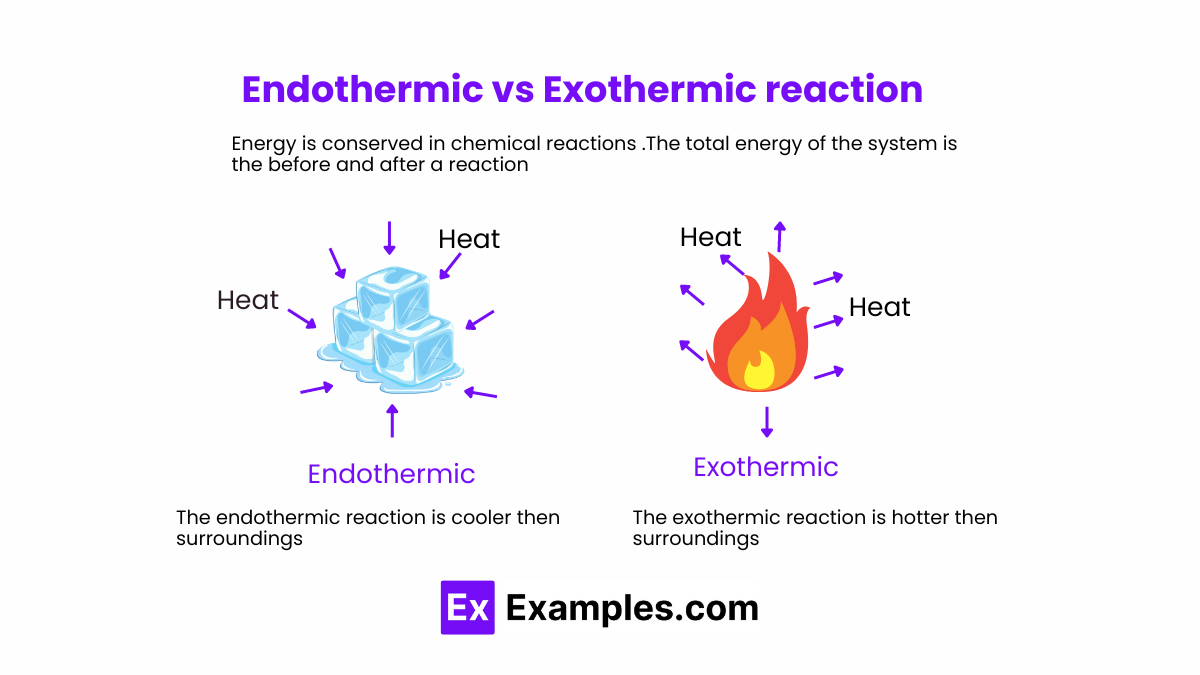
At the core, exothermic and endothermic reactions represent opposite directions of energy flow during chemical change. An exothermic reaction releases heat to the surroundings, causing temperatures to rise.In contrast, an endothermic reaction absorbs heat from its environment, often resulting in localized cooling. Defined by energy transfer, exothermic reactions satisfy the thermodynamic principle of energy conservation through outward heat flow, often described as “energy bolts out.” These reactions drive many life-sustaining and industrial processes—combustion being the most familiar. Conversely, endothermic reactions demand heat input, effectively “drawing” energy from the surroundings, much like when ice absorbs heat to melt.
The distinction can be quantified through enthalpy change (ΔH): exothermic processes have negative ΔH (releasing heat), while endothermic ones carry positive ΔH (absorbing heat). This quantifiable shift enables precise engineering applications across sectors.
Exothermic Reactions: Nature’s Heat Openers
Exothermic reactions are the engines of energy release in both living and mechanical systems.Their widespread presence reflects a universal physics principle—energy conservation in chemical form. The combustion of hydrocarbons stands as the most impactful exothermic reaction. During fuel burning—such as in gasoline engines or wildfires—carbon and hydrogen react with oxygen, producing carbon dioxide, water vapor, and substantial heat.
The process powers civilization, from internal combustion engines delivering transportation to power plants generating electricity.
“Combustion is nature’s way of releasing stored solar energy,”statements environmental scientists often cite, emphasizing exothermic reactions as key to energy availability. The reaction of methane and oxygen exemplifies this power: CH₄(g) + 2O₂(g) → CO₂(g) + 2H₂O(g) + energy (~890 kJ/mol) Similarly, the rusting of iron—an exothermic oxidation process—slowly releases heat detectable by touch, though too gradually for immediate energy recovery.
Induction and Fermentation: Hidden Exo Power Not all exothermic processes are explosive. In biological systems, fermentation releases heat in controlled doses, as seen in yeast converting glucose to ethanol and CO₂. This reaction’s mild heat output sustains bread baking and beer brewing, illustrating how exothermic chemistry shapes daily life without fanfare.
Energetic combustion in biomass also exemplifies exo behavior: burning wood in fireplaces or stoves converts stored solar energy into warmth and light—direct transformation central to heating and energy supply in off-grid settings.
Endothermic Reactions: Power from Absorption
Endothermic reactions demand energy input, making them essential in cooling systems and energy storage. Though less visible in everyday applications, their role in regulating temperature and enabling sustainable technology is profound.Photosynthesis serves as nature’s master endothermic process. Plants harness sunlight to convert carbon dioxide and water into glucose and oxygen, storing solar energy chemically: 6CO₂ + 6H₂O + light → C₆H₁₂O₆ + 6O₂ This energy-dense glucose becomes fuel for ecosystems, transforming radiant photons into stable chemical bonds.
“Photosynthesis turns sunlight into life,”scientists emphasize, underlining endothermic reactions as the gateway between solar input and biological energy use.
Other endothermic reactions, like the thermal decomposition of limestone (calcium carbonate into calcium oxide and CO₂), require extreme heat, illustrating industrial applications. In modern engineering, endo reactions power thermal energy storage systems—using molten salts to absorb solar heat during the day and release it at night, enabling 24/7 renewable energy supply. The dissolution of ammonium nitrate in cold water illustrates an endothermic effect felt physically—cooling skin when poured on a wound, a cautionary yet instructive example of heat absorption in real life.
Endothermic Chemistry in Action: Real-World Systems
The duality of exo and endo reactions enables engineered solutions across industries. Combustion engines leverage exo reactions for mobility, while endothermic processes drive innovation in sustainable energy storage and climate-responsive materials. Beyond combustion and photosynthesis, countless engineered systems depend on these thermal dynamics.Refrigeration cycles hinge on endothermic phase changes to absorb indoor heat, making homes cool and habitable. Meanwhile, phase-change materials exploit endothermic and exothermic transitions for thermal regulation in buildings and electronics. Example: Lithium-Ion Batteries and Endothermic Charging Though primarily exothermic during discharge, charging lithium-ion batteries involves endothermic absorption—lithium ions moving against gradients to intercalate into electrode structures, a process requiring energy input.
This subtle endo shift underscores the nuanced energy balance fundamental to portable power technology.
Energy Efficiency and Environmental Impact
The enthalpy changes in these reactions directly influence energy efficiency and environmental outcomes. Exothermic processes, while efficient energy releasers, often generate unwanted byproducts like CO₂, contributing to climate change.Reducing waste through catalytic optimizations remains critical. Conversely, endothermic systems offer a sustainable path: capturing and storing solar heat for later use reduces fossil fuel dependence. Innovations in endothermic thermal storage—using salts, hydrogen, or metal hydrides—represent progress toward decarbonized grids and resilient energy infrastructures.
The Continuous Cycle: Nature and Innovation in Balance
The dance between endo and exo reactions forms a continuous cycle—energy absorbed, stored, released, recycled. From the carbon-fixing exothermic rhythm of forests to the absorbing endothermic spindle of photovoltaic thermal units, nature and technology converge in thermal chemistry. This interplay not only sustains life but also propels human advancement, proving that chemistry is not just academic, but the very engine of progress.From the warmth of a flame to the subtle draw of heat in chemical storage, these reactions shape our present and future, reminding us that every spark, every temperature shift, holds the power to transform the world—one molecular exchange at a time.
/endothermic-and-exothermic-reactions-602105_final-c4fdc462eb654ed09b542da86fd447e2.png)

Related Post
Meet Dua Lipas Younger Brother Gjin Lipa
:max_bytes(150000):strip_icc()/My-BIg-Fat-Greek-Wedding-2-John-Stamos-102523-96486e1da64441b683b54bca573334a3.jpg)
My Big Fat Greek Wedding 2 Cast Unveiled: A Comprehensive Guide to Your Favorite Stars
Revolutionary Impact The Rise Of Nsfw Ai In Modern Society

