Exothermic Flames and Endothermic Chill: Harnessing Heat and Energy Through Chemical Reactions
Exothermic Flames and Endothermic Chill: Harnessing Heat and Energy Through Chemical Reactions
In the intricate dance of matter and energy, chemical reactions serve as the fundamental scripts that govern transformations—whether releasing intense heat in exothermic processes or drawing energy from the environment in endothermic ones. These two reaction types illustrate the dynamic balance between energy release and consumption, powering everything from everyday fireplaces to cutting-edge industrial applications. Understanding exothermic and endothermic reactions not only reveals nature’s thermodynamic precision but also underpins innovations in energy storage, manufacturing, and environmental science.
Unveiling Exothermic Reactions: Where Chemistry Ignites
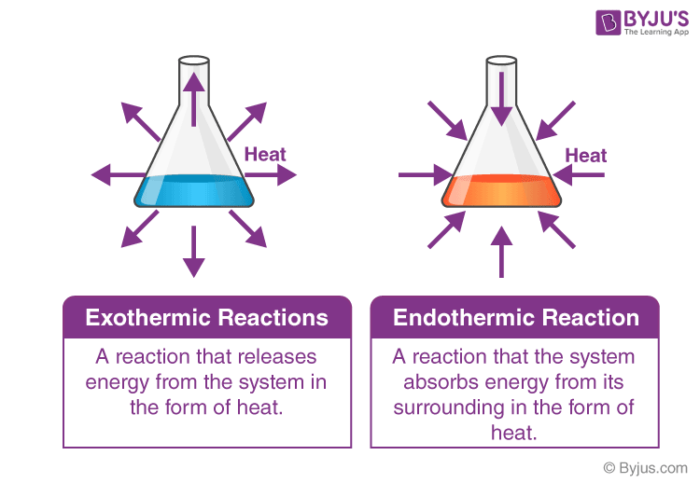
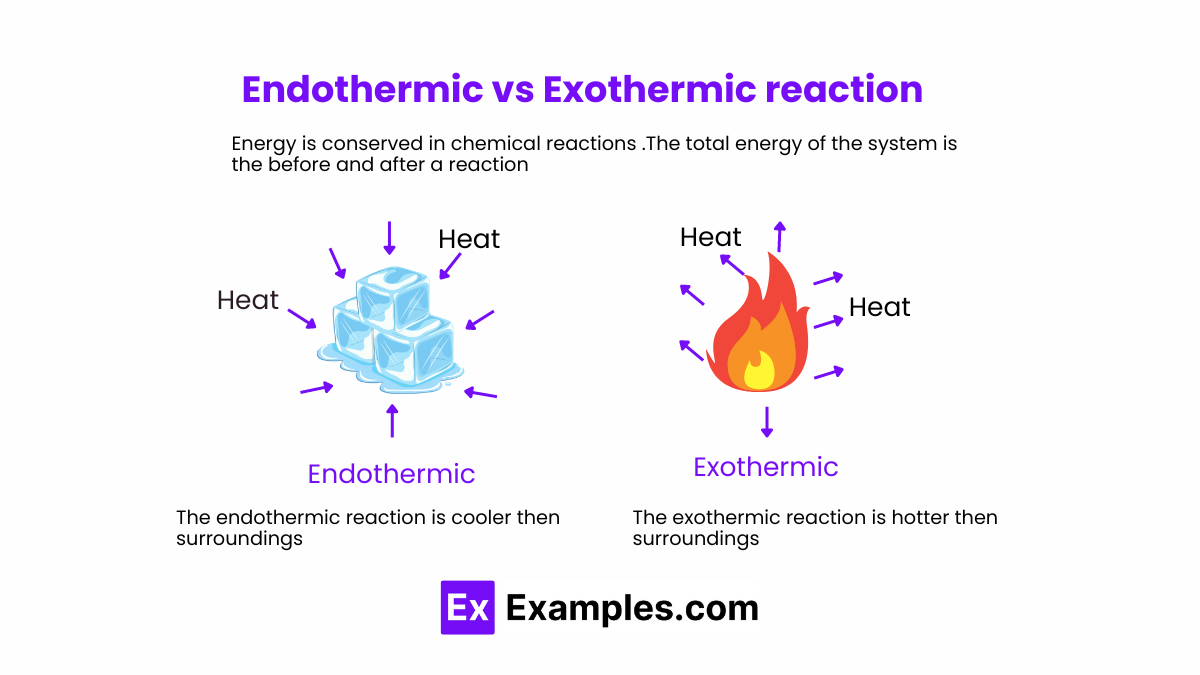
Exothermic reactions are defined by the net release of energy—primarily in the form of heat—during a chemical transformation. This energy shift manifests visibly as warmth, light, or sound, making exothermic processes both powerful and observable. The hallmark of an exothermic process is a negative change in enthalpy (ΔH < 0), meaning the system sheds more energy than it absorbs.A classic example is combustion, the reaction between a fuel and oxygen that powers engines, candles, and even home heating systems. Take methane combustion: CH₄ + 2O₂ → CO₂ + 2H₂O + Energy (released as heat and light) This reaction releases approximately 890 kJ per mole of methane—enough to sustain industrial processes and household energy needs. Similarly, the burning of hydrogen: 2H₂ + O₂ → 2H₂O + energy provides a clean alternative, gaining traction in fuel cell technology.
Fireworks offer a vivid spectacle of exothermic chemistry. The red hues come from strontium carbonate releasing oxygen, heating barium oxide in the flame. Each spark is a microburst of energy—sensible heat raising temperature, and incandescence emitting photons.
This principle extends to portable heat packs used in medicine and cold-weather gear, where iron powder reacts with oxygen in a controlled exothermic process to keep limbs warm, generating enough heat to prevent frostbite. “Exothermic reactions are nature’s way of burning cleanly and efficiently,” notes Dr. Elena Marquez, a physical chemist at the Institute for Energy Chemistry.
“They convert chemical energy into usable heat with remarkable precision, forming the backbone of thermodynamics in action.”
Key Features of Exothermic Processes> - Negative enthalpy change (ΔH < 0) confirms energy release. - Common in oxidation reactions, combustion, and molecular bond formation. - Applications span energy generation, baking, and propulsion systems.
- Heat output drives mechanical work or thermal comfort solutions.
Exothermic reactions are not limited to flames; radioactive decay also releases exothermic energy, continuously sustaining Earth’s internal heat. Yet their most widespread use lies in engineered systems where controlled heat release powers innovation.
These reactions demonstrate how chemistry transforms invisible energy into tangible thermal force, proving central to both natural processes and human technology.
Decoding Endothermic Reactions: Powering Energy Uptake
Conversely, endothermic reactions absorb energy from their surroundings to drive chemical change—a process requiring sustained input to proceed. With a positive ΔH, these reactions draw heat, often manifesting as a temperature drop or even cooling effects. Though less intuitive than exothermic processes, endothermic chemistry is vital for innovation, enabling technologies that store energy, capture carbon, and revolutionize temperature-sensitive industries.A widely recognized example is the dissolution of ammonium nitrate in water: NH₄NO₃(s) + heat → NH₄⁺(aq) + NO₃⁻(aq) + heat absorbed This reaction noticeably lowers water temperature, used in instant cold packs for medical emergency cooling. Similarly, dissolving sodium hydroxide in water is exothermic, but forms like calcium oxalate formation—used in replica casting—are endothermic, drawing heat to drive crystal growth. Sun 찬 가을 ideologies도考え지만, 실제 실험에서는 소르비톨의 수화 반응이 대표적 endothermic 사례로, 열을 흡수하며 고체로 변하며 주변 온도를 떨어뜨립니다.
“Endothermic processes absorb energy to break chemical bonds, creating conditions where energy storage or selective cooling becomes feasible,” explains Dr. James Whitmore, thermodynamics expert at the Global Energy Research Consortium. “They are the hidden engine behind technologies ranging from phase-change materials to high-efficiency refrigeration.”
From the cooling sensation of instant cold packs to the heat-trapping potential of engineered endothermic cycles, these reactions demonstrate nature’s flexibility in managing energy flow—absorbing, storing, or releasing it with deliberate control.
Core Traits of Endothermic Chemistry> - Positive enthalpy change (ΔH > 0) confirms energy absorption.
- Common in reaction-driven cooling, mineral hydration, and certain polymerizations. - Applications include thermal batteries, CO₂ capture, and cryogenic systems. - Heat input enables novel material formation and temperature regulation.
While exothermic reactions light up our world with fire and heat, endothermic processes quietly absorb energy to enable advanced thermal management and sustainable energy solutions. Together, they form a dynamic pair—each indispensable in nature and innovation—proof that energy in chemistry flows in two vital forms, shaping everything from a classroom demonstration to global power infrastructure.
The Interplay of Energy and Chemistry in Action
Understanding exothermic and endothermic reactions reveals a deeper truth: chemical transformations are not just molecular events but energy transactions that power civilization. These processes undergird technologies from indoor heating and metal smelting to carbon capture systems and next-generation batteries.As the world shifts toward sustainable energy, innovations in endothermic heat storage and high-density exothermic fuels will grow increasingly critical. “Every time we harness these reactions—whether in a furnace or a grid-scale thermal battery—we’re manipulating the fundamental laws of energy,” Marquez concludes. “Exothermic and endothermic processes are not isolated phenomena; they are the threads that weave energy science into the fabric of modern life.”
:max_bytes(150000):strip_icc()/endothermic-and-exothermic-reactions-602105_final-c4fdc462eb654ed09b542da86fd447e2.png)

Related Post

Unveiling Ullu Aap: India’s Bold Streaming App Disrupting the Digital Entertainment Landscape
The Evolution Of Kitsch Revealed: From Victorian Anachronism to Modern Cultural Icon

The Fast and Furious Cast: Star Power Behind One of Cinema’s Most Enduring Franchises
Shocking Jellybeanbrainss Leak Details: Industry Insiders Spill the Beans on a Cult Sweet’s Dark Underbelly

