Downfield vs Upfield NMR: The Hidden Force Shaping Chemical Shifts in Spectroscopy
Downfield vs Upfield NMR: The Hidden Force Shaping Chemical Shifts in Spectroscopy
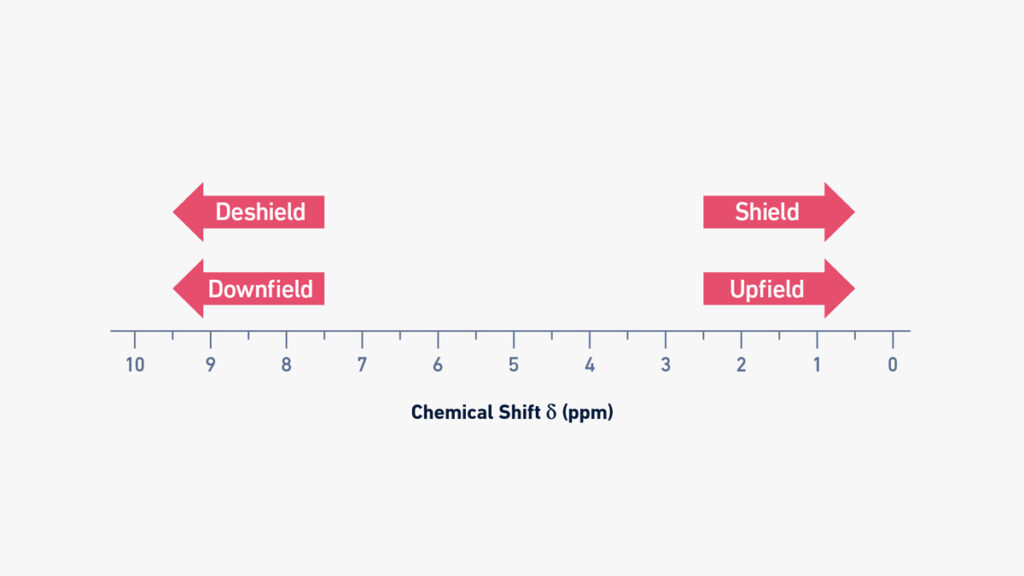
When analyzing organic compounds by NMR spectroscopy, one of the most critical distinctions lies between downfield and upfield chemical shifts—pivotal indicators that reveal a molecule’s electronic environment. Understanding why protons resonate at lower (upfield) or higher (downfield) magnetic fields transforms raw spectral data into meaningful chemical insight. This differentiation is not just academic; it is essential for accurate structural elucidation, reaction monitoring, and quality control across chemistry and biochemistry.
The fundamental reason for these shifts centers on electron density and magnetic shielding. Protons bathed in a higher electron cloud experience stronger screening, reducing exposure to the external magnetic field and thus shifting their resonance signal to higher frequencies—appearing downfield. Conversely, protons in electron-poor environments feel less shielding, increasing field exposure and pushing their signals upfield.
“The difference between an upfield and downfield resonance is not noise—it’s a whisper of electron density,” explains Dr. Lena Martinez, a senior analytical chemist at the Max Planck Institute for Molecular Physiology.
The Spectroscopic Signature: Magnetic Shielding and Electron Density
Magnetic shielding acts as nature’s protective moat around atomic nuclei.Electrons orbit the nucleus and generate tiny induced magnetic fields that oppose the external magnetic force, effectively shielding protons. In structurally simple terms: - Protons adjacent to electronegative atoms like oxygen or nitrogen are deshielded, drawing electron density away and shifting signals significantly downfield—often by 2–12 ppm. - In contrast, protons in nonpolar environments or near electron-rich regions remain shielded, manifesting as upfield peaks, typically within 0–2 ppm.
Take acetone versus ethanol as classic examples. The methyl protons in acetone resonate sharply downfield at ~2.1 ppm—reflecting deshielding from the carbonyl oxygen’s electron withdrawal—while ethanol’s hydroxyl proton appears broad and variable, usually in 1–5 ppm, influenced by hydrogen bonding and solvent effects. These positional shifts are replicateable depositions of electron distribution, making them reliable fingerprints in NMR.
Quantitative Shifts: Mapping the Chemical Space
Chemical shifts are measured in parts per million (ppm), a relative scale indexing deviation from tetramethylsilane (TMS) at 0 ppm. - **Upfield peaks (>2 ppm):** Highest electron density, minimal deshielding. Examples include –CH3 (0.8–1.5 ppm), alkanes, and protons in aliphatic chains.- **Middle field (1–2 ppm):** Moderately shielded, found in alkenes (e.g., propene at ~1.6 ppm) and methylenes adjacent to few electronegative groups. - **Downfield shifts (<1 ppm):** Low electron density, strong deshielding. Aromatic protons average 6–8 ppm, carboxylic protons at 10–12 ppm, and aldehyde –CHO at ~9–10 ppm—among the strongest shifts.
These ranges form a predictive framework. For instance, the downfield position of benzene’s aromatic ring protons reveals conjugated π-electron density that stabilizes the molecule, while an aldehyde’s high-field deshielding signals its oxidized state with precision.
Factors Influencing Shifts: Beyond Basic Structure
While functional groups dominate, multiple factors subtly modulate chemical shifts, complicating but enriching interpretation.- **Electronegativity:** Fluorine, chlorine, and oxygen pull electron density, deshielding nearby protons. - **Hydrogen bonding:** Hydroxyl and amine protons stretch high due to H-bonding—seen in alcohols and carboxylic acids near 10–12 ppm. - **Magnetic anisotropy:** π-electron systems in aromatic rings or double bonds induce localized shielding changes; benzene’s entire ring system spreads over 6–8 ppm.
- **Temperature and solvent:** Dynamic equilibrium, hydrogen bonding strength, and polarity shift peak positions subtly—essential considerations in kinetic and solution NMR. These nuances mean downfield signals aren’t always straightforward; a proton near multiple deshielding influences may shift non-invariantly, demanding careful overlapping spectral analysis.
Applications in Science and Industry
The dichotomy of downfield and upfield shifts powers diverse applications across research and manufacturing.- In pharmaceutical development, tracking downfield shifts in active ingredients or impurities enables real-time quality control, ensuring drug purity and stability. - Metabolomics relies on NMR’s chemometric power—subtle downfield echoes in biological fluids reveal metabolic pathways and disease biomarkers, such as elevated lactate (δ ~2.1–2.4 ppm) in anaerobic conditions. - Materials science probes conductive polymers and catalysts via downfield peaks in pyridine rings or metal-bound protons, assessing charge distribution and reactivity.
Clinically, magnetic resonance imaging (MRI) harnesses TMS-related shifts: fat’s downfield signal (~–4 ppm) contrasts with water (~3.5 ppm), allowing fat-suppressed imaging critical for diagnosing tissue abnormalities.
Understanding downfield versus upfield NMR behavior equips scientists with a precise lens to decode molecular architecture. Far more than a spectral curiosity, the contrast between shielded and deshielded protons underpins modern analytical chemistry, bridging the gap between electron density and chemical identity.
As analytical instruments grow more sensitive, these once-invisible shifts emerge as decisive tools—transforming data into decisive insight.



Related Post
They’re Not Who You Think They Are: Unveiling the Truth Behind the Iconic Sports Figure Often Reimagined
Code For Transfer in First Bank: Revolutionizing Digital Transfers with Speed, Security, and Simplicity

Is Z-Library Safe? The Full Risk Assessment of the World’s Most Controversial Book Archive
Revolutionizing Healthcare Simulation with Arturo Pérez and Shadow Health: Transforming Patient Learning Through Immersive training

