Defining a Subscript in Chemistry: The Silent Notation That Shapes Molecular Precision
Defining a Subscript in Chemistry: The Silent Notation That Shapes Molecular Precision
In the intricate language of chemistry, every symbol carries weight—often invisible at first glance, yet pivotal to clarity and accuracy. Among the most foundational notations, the subscript serves as a silent architect of molecular identity, transforming abstract symbols into precise strings that define atomic composition and stoichiometry. Defined as a small number placed to the right of a chemical symbol—most commonly on the right side of an atom or element—it signals the quantity of atoms in a given molecule, ion, or formula.
Understanding the definition of a subscript is essential not only for mastering basic chemical formulas but also for navigating advanced concepts in molecular reactions, stoichiometric calculations, and structural chemistry. The subscript’s role extends far beyond mere notation; it is the backbone of chemical communication, enabling scientists to convey exact atomic ratios with unambiguous faith. Without subscripts, the formula calcium carbonate (CaCO₃) would imply only a vague grouping of elements, losing the critical insight that one calcium atom bonds with one carbon atom and three oxygen atoms.
This precision ensures that chemists can reliably predict molecular behavior, design drug compounds, balance reactions, and synthesize materials with confidence.
Decoding the Subscript: Definition and Function in Chemical Formulas
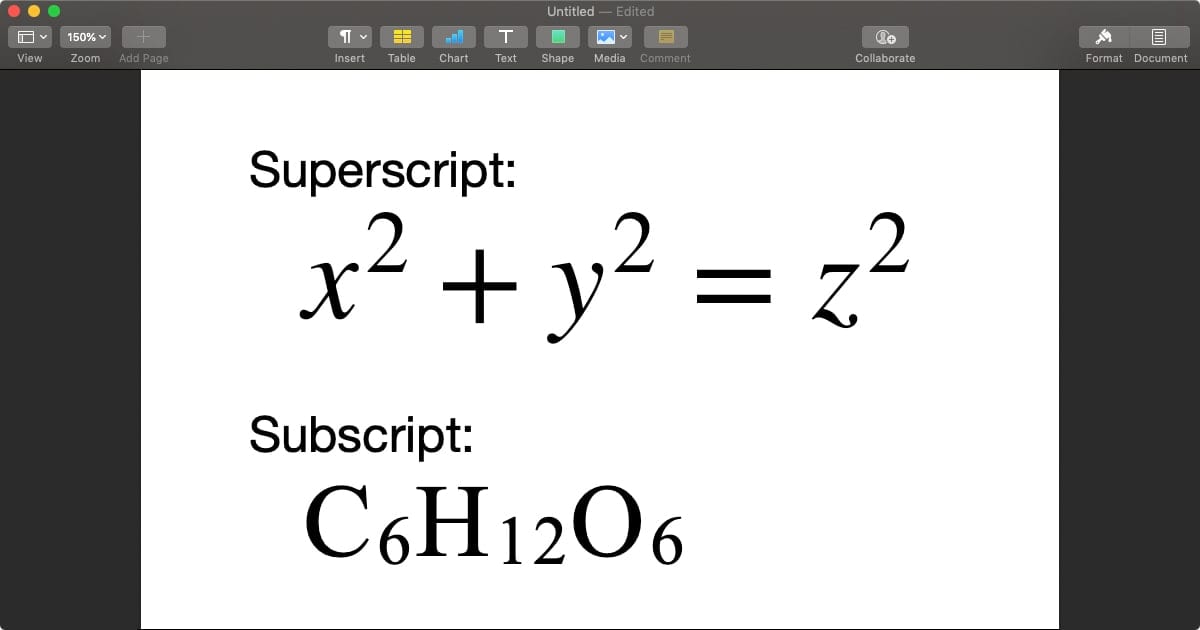
A subscript appears as a small number immediately adjacent to a chemical symbol—typically on its lower right—denoting the count of identical atoms in a chemical entity. Its presence or absence determines whether a formula refers to an element, simple molecule, ion, or compound.For instance, sodium chloride (NaCl) uses subscript 1 implicitly for each atom, reflecting a 1:1 ratio, while diatomic bromine (Br₂) uses a superscript 2 to indicate two atoms covalently linked. This seemingly simple convention encodes vital information: the stoichiometric ratio between elements that governs chemical behavior. Subscripts are integral in: - Atomic molecules (e.g., O₂, H₂O, CO₂): specifying bonding patterns - Ionic formulas (e.g., Na⁺ and Cl⁻ forming NaCl): defining total charge neutrality - Polyatomic species (e.g., NO₃⁻, SO₄²⁻): conveying complex ion structure - Complex compounds (e.g., Fe₂O₃, Al₂(SO₄)₃): clarifying metal and ligand counts As chemist Peter Atkins once stated, “The power of chemistry lies not just in reactions, but in the language that describes them—starting with the subscript’s quiet yet definitive role.” This precision allows chemists to decode everything from the structure of a vitamin molecule to the mechanics of a catalytic converter in a car.
Each subscript position serves as a firm anchor in molecular nomenclature. In a diatomic hydrogen molecule (H₂), the subscript 2 denotes two hydrogen atoms forming the simplest stable elemental bond. In metal oxides like MgO, the subscript 2 confirms one magnesium ion paired with two oxygen atoms, crucial for predicting ionic charges and lattice stability.
In polyatomic ions such as phosphate (PO₄³⁻), the subscript 4 reflects the four oxygen atoms bound to a central phosphorus atom with a net -3 charge, directly influencing how the ion interacts in solutions and special reagents. Defining a subscript as “a numerical indicator of atomic count in a chemical entity” captures its essence—but deeper significance emerges when analyzing real-world applications. Consider ammonium nitrate (NH₄NO₃): the numbers 1 and 3 clarify that one nitrogen and four hydrogen atoms bind with three oxygen atoms, forming a compound critical in fertilizers and explosives.
Without accurate subscripts, such formulas become ambiguous, risking errors in synthesis, dosage calculations, or theoretical modeling.
Historical Evolution and Standardization of Subscripts in Chemical Formula Writing
The formal use of subscripts in chemistry evolved gradually, reflecting broader advancements in chemical understanding. Early alchemists and 18th-century chemists gradually moved from qualitative descriptions toward standardized notations to express empirical formulas.Anonymous yet pivotal contributions from scientists like Antoine Lavoisier and Jöns Jacob Berzelius helped cement symbolic rules, including consistent use of subscripts to denote atomic ratios. By the 19th century, the modern chemical formula conventions—especially subscripts—gained widespread adoption, driven by the need for reproducibility and clarity across laboratories and publications. Today, the International Union of Pure and Applied Chemistry (IUPAC) provides rigorous guidelines that enforce correct subscript placement, eliminating ambiguity in formulaic communication worldwide.
The standardization ensures universal comprehension: - Atomic symbols remain consistent (e.g., H for hydrogen, O for oxygen) - Subscripts follow strict rightward placement and concise numerical use (rarely beyond 4 in isolated formulas) - Ionic charges and polyatomic configurations incorporate subscripts and charges when necessary (e.g., Al₂(SO₄)₃, where the 3 indicates three sulfates per aluminum ion) This system parallels developments in digital data representation—where unambiguous symbols enable flawless computation, data exchange, and machine readability—underscoring the subscript’s role as a foundational element in scientific precision.
In biochemical pathways, this precision becomes indispensable. The subscript-enabled formula glucose (C₆H₁₂O₆) precisely communicates six carbons, twelve hydrogens, and six oxygens—critical for modeling cellular respiration or designing targeted metabolic drugs.
Ultimately, the definition of a subscript transcends mere notation: it is the silent guardian of accuracy in a discipline where atomic detail dictates macroscopic outcomes. Every subscript anchors molecular identity, enabling everything from textbook teaching to industrial innovation. As chemistry continues to advance—from nanomaterials to synthetic biology—the subscript remains an indispensable tool, deceptively small yet monumental in shaping scientific clarity and progress.



Related Post
Mary Beth Evans Bio Wiki Age Husband Days Of Our Lives As The World Turns and Net Worth

Isabel Lawrence Wkyc: The Life, Legacy, and Pioneering Education Journey of a Welsh Media Icon

The Power Behind Cos Sin Tan: Unlocking Complex Trigonometry for Engineering and Science
X Chromosome Y: The Genetic Architect Shaping Male Development and Beyond

