Define Volume in Chemistry: The Unseen Measure That Shapes Every Reaction
Define Volume in Chemistry: The Unseen Measure That Shapes Every Reaction
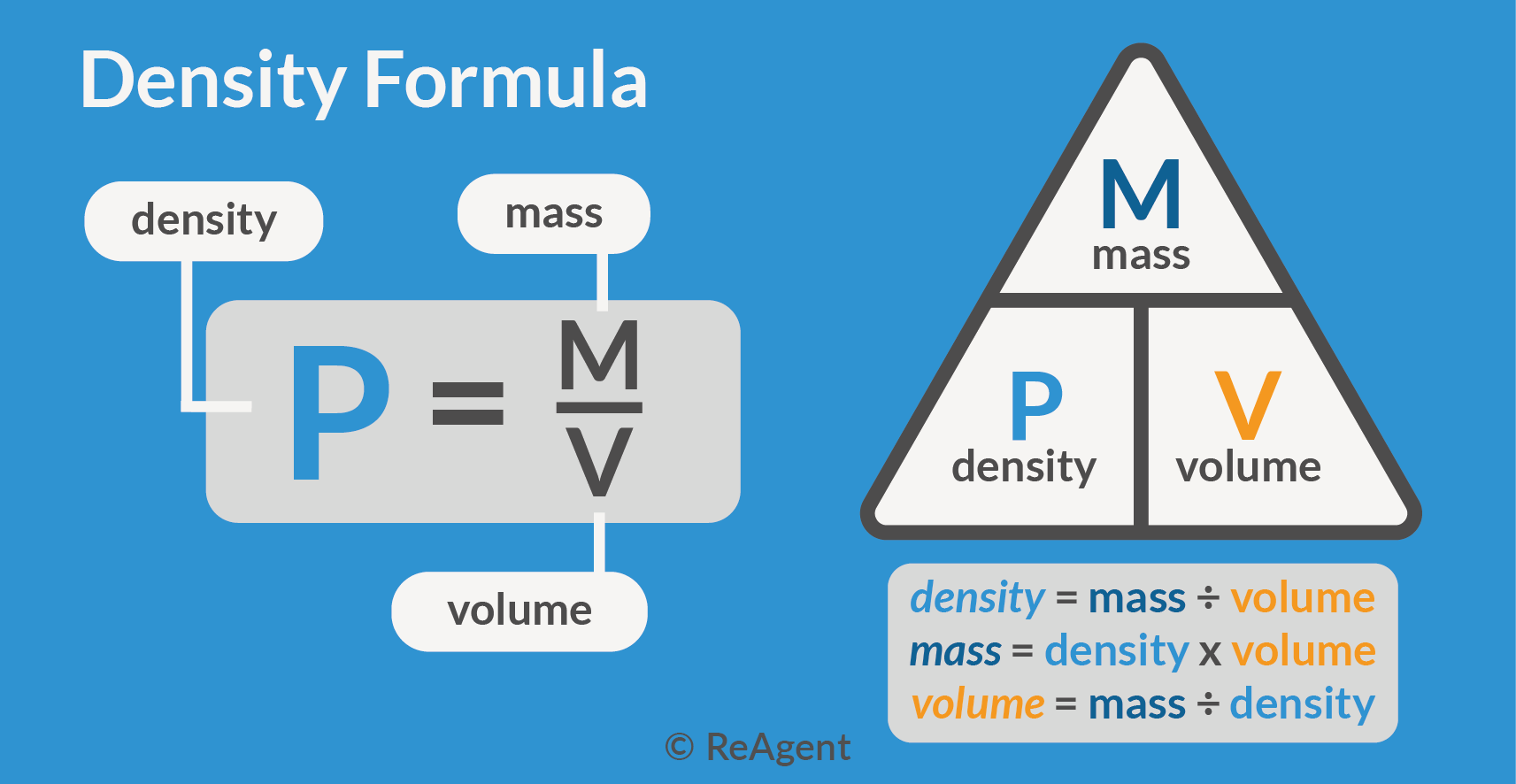
Volume in chemistry is far more than a simple number on a label—it is a foundational concept that governs how substances interact, react, and behave under different conditions. Defined as the three-dimensional space occupied by a substance, volume provides critical insight into molecular density, concentration, and reaction kinetics. In chemical systems, volume acts as a bridge between macroscopic measurements and microscopic behavior, enabling precise control in laboratories, industrial processes, and environmental modeling.
At its core, volume is measured in liters (L), cubic meters (m³), or milliliters (mL), and its accurate determination is essential across scientific and engineering domains. In liquid solutions, volume directly influences molarity—the number of moles of solute per liter of solution—dictating reaction rates and product yields. For gases, volume plays an even more dramatic role, governed by the ideal gas law (PV = nRT), where pressure, temperature, and temperature dictate the spatial distribution of molecular particles.
Understanding volume allows chemists to scale reactions safely, optimize storage, and predict phase changes.
The Role of Volume in Solution Chemistry
In aqueous and non-aqueous solutions, volume determines concentration—a cornerstone of quantitative analysis. Concentration, defined as the amount of solute per unit volume of solution, enables scientists to standardize experimental conditions. For instance, preparing a 0.5 M sodium chloride solution requires not only knowing the mass of NaCl but also precisely measuring 500 mL of water to achieve the correct dilution.Deviations in volume lead to errors in stoichiometric calculations, potentially invalidating reaction stoichiometry and compromising experimental integrity.
A classic example lies in titrations, where accurate volume measurement of titrants ensures reliable endpoint detection. If 25.0 mL of 0.1 M HCl is used to neutralize a base, the volume—recorded to the nearest 0.1 mL—directly affects the moles of H⁺ available to react.
Even minor inaccuracies in pipette or burette readings propagate through calculations, risking affect any analysis built upon that base.
Volume also governs dilution effects. When concentrating a stock solution by reducing its volume, the solute concentration increases proportionally (C₁V₁ = C₂V₂), a relationship governed by volume conservation. This principle underpins safe handling of hazardous chemicals, enabling dilution from highly concentrated to biologically inert forms in pharmaceutical manufacturing and laboratory preparation.
Volume and Gas Behavior: From Idea to Industry
When gases are involved, volume reveals dynamic interactions governed by pressure, temperature, and the amount of substance.The ideal gas law — PV = nRT — positions volume as a measurable determinant of molecular behavior. At constant temperature and moles (n), volume is inversely proportional to pressure, a concept leveraged in pressurized storage tanks and gas chromatography. In industrial settings, understanding gas volume at standard temperature and pressure (STP—0°C, 1 atm) allows precise calibration of pipelines, breathing apparatuses, and chemical reactors.
Gases expand or contract dramatically with changes in pressure and temperature, making volumetric precision essential in aviation, deep-sea equipment design, and cryogenics. For example, a 100-liter methane tank at room temperature occupies vastly more volume under vacuum conditions, demanding careful volume control to maintain operational safety and safety margins. Similarly, scuba divers manage lung gas volume through breath-hold and exhalation techniques, illustrating how volume influences human physiology at atmospheric extremes.
In mixing gases, volume helps predict partial pressures via Dalton’s Law, where total pressure equals the sum of individual component pressures. This principle ensures accurate modeling of atmospheres in respiratory systems, combustion chambers, and planetary atmospheres. Every volume measurement thus contributes to safer and more efficient chemical engineering and environmental monitoring.
Volume in Reaction Dynamics and Stoichiometry
Volume is indispensable in stoichiometric calculations, where mole ratios depend on solution or gas volumes under standardized conditions.In laboratory titrations, reaction volumes determine equivalence points: mixing equal volumes of standard solutions often signifies complete neutralization, but deviations necessitate recalibration of volume readings.
Consider the neutralization of 50.0 mL of 0.2 M H


Related Post

The Craft of Mastery: Decoding the New New York Times Spelling Bee Answers and What They Reveal About Language, Creativity, and Cognitive Challenge

Bryce Gheisar: The Rising Star Dominating Rotten Tomatoes with Blind Confidence and Pinpoint Performances

Casio 6250 Reimagines Chord Dawai Asmara Lagu Rhoma Irama: A Rhoma Irama Tribute in Unrecognized Ballad Notation
Kayla Moody Net Worth Career Family Boyfriend

