Decoding Thermodynamics: The Critical Role of Delta G in Predicting Reaction Spontaneity
Decoding Thermodynamics: The Critical Role of Delta G in Predicting Reaction Spontaneity
Could the key to understanding whether a chemical reaction will proceed naturally under given conditions lie hidden in a single, powerful equation? Yes — Delta G, the Gibbs free energy change, offers a precise thermodynamic metric that determines reaction spontaneity, driving innovation across chemistry, biochemistry, and engineering. By explaining the De ลงไป
The Gibbs Free Energy Equation: The Mathematical Core of ΔG
At the heart of predicting chemical behavior under constant temperature and pressure lies the equation ΔG = ΔH – TΔS — a concise yet profound expression defining Gibbs free energy change.This formula, attributed to Paraguayan scientist Josiah Willard Gibbs, integrates three fundamental thermodynamic quantities: ΔH (enthalpy change), T (absolute temperature in kelvins), and ΔS (entropy change). Each component reveals distinct aspects of molecular disorder and energy flow. Enthalpy captures heat exchange during a reaction, temperature scales the system’s thermal energy influence, and entropy quantifies randomness in molecular arrangements.
When combined, they form a decisive criterion: whether ΔG is negative, positive, or zero dictates reaction feasibility.
Understanding ΔG requires unpacking its components. Enthalpy change (ΔH) reflects bond formation and breaking — exothermic reactions release energy, often favoring spontaneity, while endothermic processes absorb heat, potentially resisting spontaneity unless compensated by entropy.
Entropy change (ΔS), expressed per mole, measures shifts in molecular order; systems evolving toward higher disorder (increased randomness) contribute negatively to ΔG, especially at elevated temperatures. Temperature (T) acts as a multiplier, amplifying entropy’s impact when large. The product TΔS introduces dynamic flexibility into the equation, enabling reactions to proceed even with limited enthalpy favorability if entropy gain is substantial.
odontash>
When ΔG Meets Reality: Predicting Reactions from First Principles
Consider steel corrosion: iron oxidizes in moist air (ΔH slightly negative, ΔS positive due to increased oxide disorder), making ΔG slightly negative under ambient conditions — a thermodynamic urge toward rust. Yet, the reaction rate depends on kinetics, showing ΔG predicts feasibility, not speed. In biochemical systems, ATP hydrolysis exemplifies near-zero ΔG (equilibrium), powering cellular functions through carefully balanced energy release.Enzymes modulate local conditions, effectively steering TΔS toward driving endergonic (ΔG > 0) reactions.
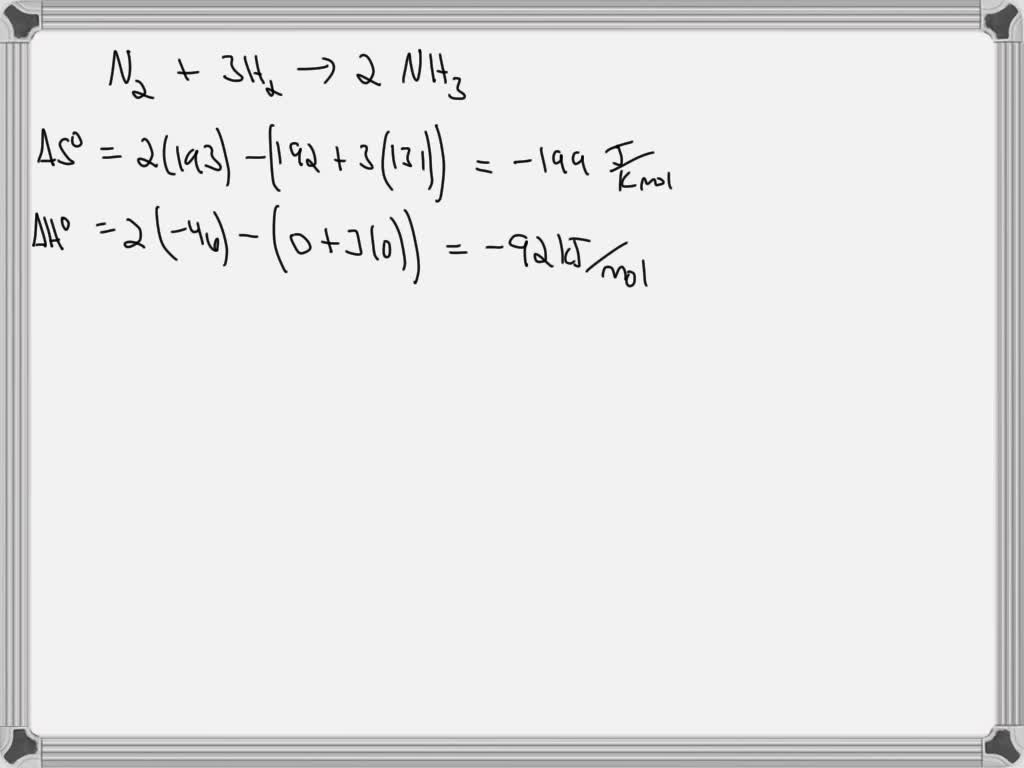
One of the most compelling applications unfolds in material science. Consider synthetic ammonia production via the Haber-Bosch process.
The reaction N₂(g) + 3H₂(g) → 2NH₃(g) is exothermic (ΔH < 0), but ΔS is negative due to fewer gas molecules. Thus, low temperatures favor spontaneity thermodynamically, yet industry balances yield and rate by using moderate temperatures (~450°C) and high pressure. Here, ΔG becomes a critical decision tool: optimizing temperature so ΔG is sufficiently negative to proceed while minimizing decomposition back to reactants.
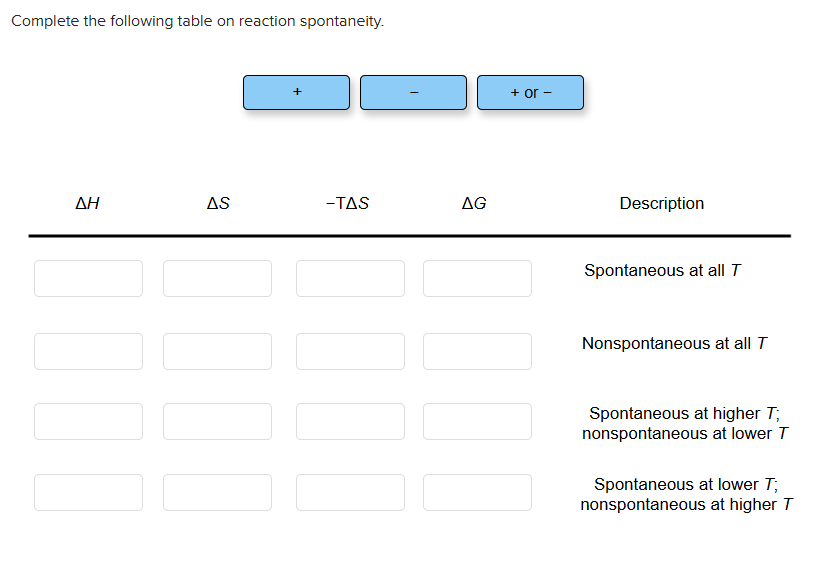
- ΔG = 0: System at equilibrium; no net change.
- ΔG < 0: Spontaneous reaction proceeds forward.
- ΔG > 0: Non-spontaneous; reaction favors reverse under standard conditions.
Measuring ΔG analytically often relies on standard conditions, with ΔG° representing free energy change under reference entropy and enthalpy, plus TΔS°. Real-world applications instead use actual ΔG via:
Electrochemical Cells: Measuring cell potential (E) yields ΔG = –nFE, where n is moles of electrons and F is Faraday’s constant. This direct link enables batteries and industrial electrolysis optimization.
Computational Thermodynamics: High-performance modeling calculates ΔG using quantum chemistry or statistical mechanics, predicting reaction outcomes without lab trials — vital in drug design and green chemistry.
Biochemical Assays: In living systems, ΔG impredicts metabolic flux; deviations signal pathological states, informing diagnostics and targeted therapies.
Entropy’s role in ΔG extends beyond science into philosophy. It encapsulates nature’s drive toward disorder — a universal tendency that thermodynamics quantifies.
Yet, nature also crafts pockets of gradient-driven order. Photosynthesis defies entropy locally, harnessing sunlight to synthesize glucose (ΔG < 0) from CO₂ and H₂O. Such processes reveal ΔG governs boundaries — between order and chaos — shaping planet-scale cycles from ocean chemistry to cellular energetics.
Engineers leverage ΔG insights to design sustainable technologies. Carbon capture systems, for instance, manipulate


Related Post

Mila Ruby Tits: Revolutionizing Memory, Learning, and Neural Plasticity in Neuroscience
Juan Jose Soto: Decoding the Legacy and Impact of Baseball's Dynamic Outfielder

