Decoding the Periodic Table: Ionic Charges Explained Through Elemental Fire And Facets
Decoding the Periodic Table: Ionic Charges Explained Through Elemental Fire And Facets
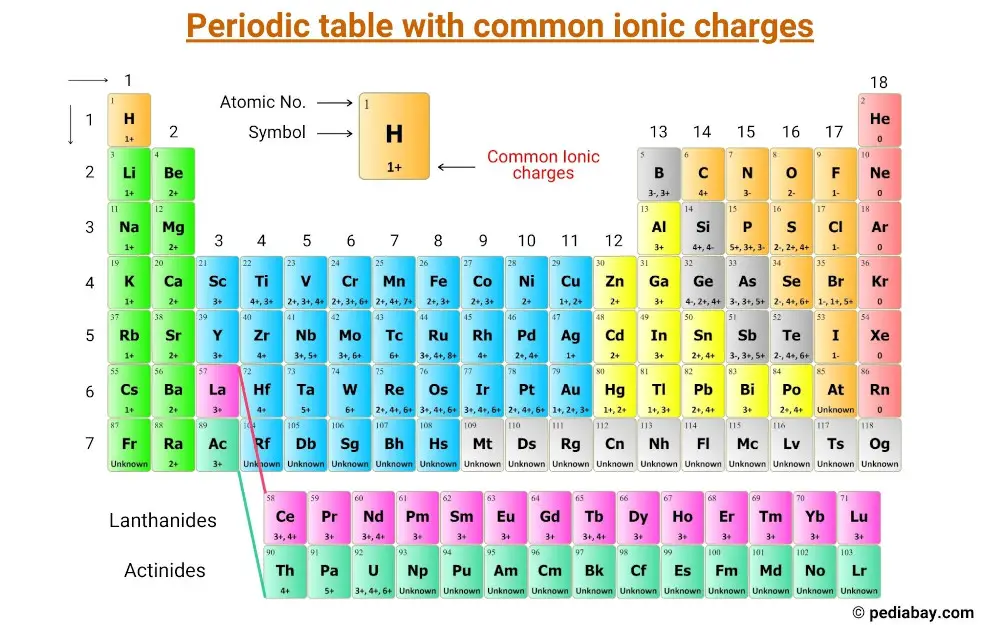
Navigating the Periodic Table reveals a hidden order beneath elemental symbols—shifting ions defined not just by their atomic numbers, but by precise ionic charges that dictate their chemical behavior. From sodium’s swift +1 to chromium’s complex +3 and –2, each charge reflects core principles of electron transfer and stability. Understanding these ionic charges empowers chemists, educators, and students to predict reactivity, design compounds, and unlock life’s molecular mechanisms.
This deep dive explores the most common ions across the table, their ascending and descending charge patterns, and their real-world significance—with clarity, precision, and intent.
Ionic Charges: The Language of Electron Exchange
Ionic charges emerge from atoms gaining or losing electrons to achieve a full valence shell, aligning with the octet rule. When atoms lose electrons, they become positively charged cations; electrons gained results in negatively charged anions. This fundamental charge transfer enables atomic recombination into stable ionic compounds, driving everything from salt formation to biological signaling.
As physicist Richard Feynman once noted, “Electrons are the messengers of chemistry”—and their charge determines how elements interact across the Periodic Table.
The periodic arrangement reveals predictable trends: from left to right, successive elements tend to lose increasing numbers of electrons, forming cations with higher positive charges, while right to left, elements gain electrons more readily, forming anions with higher negative charges. Transition metals and lanthanides complicate this due to variable oxidation states, introducing multiple ionic forms for a single element. Yet the core principle remains: ionic charge dictates bonding patterns, solubility, and reactivity.
Key Cations Across the Periodic Table
Cations dominate transitional and main-group elements, illustrating gradual loss of valence electrons.
Alkali Metals (Group 1): These reactive metals shed one electron effortlessly, achieving +1 charge. Lithium (Li⁺) initiates the series, followed by sodium (Na⁺), with+1 charge increasing down the group due to decreasing nuclear hold on outer electrons.
Alkaline Earth Metals (Group 2): Two-electron loss defines calcium (Ca²⁺), magnesium (Mg²⁺), and barium (Ba²⁺), each gaining a +2 charge to stabilize their twice-heavily bound valence shells.
Transition Metals: Flexible oxidation states allow a range of charges. Iron transitions from +2 to +3; copper from +2 to +1 and +2. Zinc consistently forms Zn²⁺, balancing d-orbital stability.
Anions: Electron Gain and Multiplicity
Most nonmetals gain electrons to form anions, typically achieving -1, -2, and in molecules like O²⁻ or O²⁻. Halogens dominate this group, thriving to attain -1 for a stable octet.
Halogens (Group 17): Fluorine (F⁻), chlorine (Cl⁻), bromine (Br⁻), and iodine (I⁻) all carry a -1 charge, their electron affinity driving compact ionic lattices and high reactivity—halogens form strong ionic bonds with metal cations.
Transition Metal Anions and Variable Charges
Beyond the standard -2 charge, transition metals exhibit complex +1 to +3, sometimes +2, ex reflecting d-electron cancellation. Chromium defies simplicity: Cr³⁺ carries +3, but Cr⁶⁺ yields –2, depending on complex ligands. Manganese varies from +2 to +7 in biologically critical enzymes.
These shifting charges illustrate the role of ionization energy, electron configuration, and coordination chemistry in defining oxidation states.
Periodic Trends and Charge Patterns Explained
The placement of elements governs their ionic potential. Moving left across a period increases positive cations due to rising effective nuclear charge; moving down enhances negative anions as outer shells expand and electron attraction weakens slightly.
Critical Observations:
- Ion +1 most common among nonmetals (F⁻, Cl⁻


/PeriodicTableCharge-WBG-56a12db23df78cf772682c37.png)
Related Post

The Collapse That Shook a Nation: Unraveling Japan’s Bubble Burst and Its Lasting Lessons
Face Card Meaning: Unlocking the Power Behind Iconic Cards in Culture, Cards, and Code
Airport Fort Lauderdale: The Gateway Beneath the Glitz of Broward’s Skyline
Monique Comedian Net Worth: From Local Stage to National Stardom — A Comedian’s Ascent and Financial Rise

