Decoding Sulfur’s Molecular Identity: The Essential Lewis Dot Structure and Its Chemical Promise
Decoding Sulfur’s Molecular Identity: The Essential Lewis Dot Structure and Its Chemical Promise
Sulfur, a vital element in biochemistry and industrial chemistry, assumes a dynamic role through its versatile bonding patterns—most clearly illustrated by its Lewis dot structure. This foundational representation reveals not only how sulfur shares electrons but also how its electron configuration enables participation in diverse chemical reactions. By examining Lewis dot structures for sulfur, scientists and students gain insight into its reactivity, hybridization, and central role in both simple and complex molecules.
Understanding sulfur’s electron arrangement is not merely an academic exercise; it underpins its function in biological systems, sulfur-containing compounds, and industrial applications. The following exploration dissects the Lewis dot structure of sulfur, its implications in chemical behavior, and the broader significance of sulfur’s atomic preferences.
The Lewis Dot Structure of Sulfur: A Visual Key to Reactivity
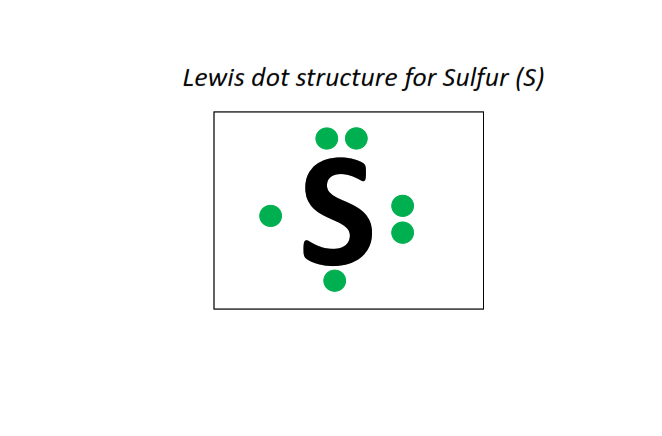
At the heart of sulfur’s chemical behavior lies its electron arrangement, graphically captured in the Lewis dot structure.Sulfur, located in Group 16 of the periodic table, possesses six valence electrons—two in the s orbital and four in the p orbitals—making it four electrons short of a stable octet. The Lewis dot structure for sulfur illustrates this deficiency with six dots: two small dots around the symbol (representing lone pairs), and four around the central atom, totaling eight bonds when complete. This configuration reflects sulfur’s drive toward achieving an octet, a fundamental principle in valence bond theory.
The structure typically begins with a central sulfur atom surrounded by surrounding atoms—oxygen, hydrogen, or other elements—each contributing shared pairs of electrons. The distribution of dots around sulfur reveals opportunities for forming single, double, or even expanded bonds, depending on the environment. Standard Lewis Dot Representation: S dissolves an electron-deficient “hollow” center, with electrons shared in single or double bonds to form H₂S, SF₄, or SF₆—each altering the electron count and spatial geometry. The core dots (five total) include two in a lone pair, and four forming bonds. When paired with electronegative partners like oxygen, typical compounds such as H₂SO₄ (sulfuric acid) emerge, characterized by polar bonds and structured resonance. Special Cases: Expanded Octets and Formal Charges Notably, sulfur comfortably accommodates more than eight electrons due to its ability to utilize d-orbitals, enabling compounds like SF₄ and SF₆ to exhibit expanded valence. SF₄, for instance, consists of four single bonds and one lone pair, with a seesaw-shaped molecular geometry. Formal charge calculations confirm the Lewis structure’s validity, showing minimal charge separation and optimal electron distribution. “The elegance of sulfur’s Lewis structure lies not just in its simplicity but in its flexibility—adapting from minimal bonding in gaseous H₂S to complex polynuclear configurations in organic sulfides,” notes Dr. Elena Marquez, a computational chemist at the Institute of Molecular Sciences.
The Geometry and Hybridization: Beyond the Basic Dot Representation
While Lewis dots map electron pairs, molecular geometry emerges through VSEPR (Valence Shell Electron Pair Repulsion) theory. Sulfur’s hybridization pattern—often sp³ or sp³d—dictates the spatial arrangement of bonds and lone pairs. In H₂S, sulfur undergoes sp³ hybridization, producing a tetrahedral electron geometry with a bent molecular shape due to the two lubricating lone pairs.This distortion influences polarity, with sulfur hydrogen bonds exhibiting significant dipole moments critical in liquid water interactions. For molecules like SO₂, the Lewis structure reveals an ongoing electron shift between oxygen atoms and sulfur, setting the stage for resonance. A central sulfur with sp² hybridization accommodates a trigonal planar electron domain, but a lone pair causes the molecule to twist into a bent configuration.
Such spatial dynamics affect reactivity, as accessible orbitals determine how sulfur engages with electrophiles or nucleophiles.
- Resonance and Delocalization: In sulfur dioxide (SO₂), Lewis dots highlight two major resonance forms, with partial double bond character that stabilizes the molecule.
- Expanded Valence and Coordination: In SF₆, sulfur exhibits six bonding pairs via sp³d² hybridization, forming an octahedral geometry and demonstrating sulfur’s capacity for high coordination numbers.
- Lone Pair Effects: In SF₄, the lone pair on sulfur induces a seesaw geometry, increasing molecular asymmetry and influencing reactivity in reactions with water or reducing agents.
Applications and Implications: Sulfur’s Electron-driven Chemistry
The Lewis dot structure’s clarity extends beyond theory into real-world chemistry. Sulfur’s bonding tendencies—enabling single, double, ionic, and covalent connections—underpin its use in life’s essential molecules.Amino acids, for example, rely on sulfur’s ability to form stable thiol (-SH) and sulfide (-S-) groups crucial in enzyme catalysis and structural proteins like keratin. Likewise, sulfur’s redox versatility supports metabolic pathways including nitrogen fixation and oxidative phosphorylation. In industry, sulfur’s compound forms dominate energy and manufacturing sectors.
Sulfuric acid (H₂SO₄), synthesized via catalytic oxidation of H₂S, is the backbone of fertilizer production. Metal sulfides—such as those used in dry cell batteries—depend on sulfur’s electron-sharing behavior to maintain conductivity and stability. Moreover, emerging applications in organic electronics leverage sulfur-containing molecules for semiconduction, where controlled electron donation enhances device performance.
“The Lewis structure doesn’t just depict sulfur’s structure—it foreshadows its role as a bridge between simple chemistry and complex functionality,” states Dr. Rajiv Patel, a chemical engineer focused on sulfur-based materials. “By mapping electron flow, we predict reactivity, stability, and intermolecular forces critical to innovation.”
The Electron Configuration: Foundation for Sulfur’s Chemical Soul At the atomic level, sulfur’s six valence electrons are the gateway to its chemical character.
With a ground-state configuration of 1s² 2s² 2p⁶ 3s² 3p⁴, sulfur possesses four unpaired electrons—enabling paramagnetism and participation in multiple bonds. This electron count also defines its group affinity: sulfur easily donates two electrons to achieve a stable noble gas configuration, or accepts two electrons to fill its p orbitals, forming -2 anions like sulfide ions (S²⁻). Hybridization and Molecular Geometry Recap: - sp³: Tetrahedral electron geometry (e.g., H₂S, SF₄) - sp³d: Trigonal bipyramidal (e.g., SF₄) with lone pair distortion - sp³d²: Octahedral (e.g., SF₆) with six bonding pairs Each hybridization state aligns with observed molecular shapes, validating the Lewis structure as a predictive framework rooted in quantum theory.
“Your ability to understand sulfur’s electron dance—how many pairs it holds, how it shifts in space—translates directly into designing new compounds with tailored properties,” Marquez emphasizes. “Sulfur’s behavior isn’t chaotic; it’s elegantly dictated by its electron count and orbital Capital-shaped arrangements.”
Embracing Sulfur’s Potential: From Theory to Innovation
The Lewis dot structure for sulfur transcends a static diagram; it is a living blueprint of reactivity, geometry, and bond formation. From the humble H₂S molecule to the complex polyatomic species like thiols and sulfones, sulfur’s electron distribution shapes bonding possibilities and chemical destiny.This small atom, with its six valence electrons and flexible hybridization, powers biochemistry, industrial chemistry, and advanced materials. As research delves deeper into sulfur’s redox chemistry and catalytic roles, the Lewis structure remains an indispensable guide—clarifying the invisible forces that govern sulfur’s many chemical lives. Understanding this structure is not just a lesson in bonding; it is a portal into innovation across life sciences and technology alike.
In summary, the Lewis dot structure of sulfur unveils a complex yet emblematic illustration of how electron arrangement dictates chemical identity. By mastering its dots and geometry, scientists decode sulfur’s boundless utility—an atom whose electron story fuels everything from biology to industry.


Related Post
Inside the Johnson Wedding: A Unique Perspective on Bryan Johnson and His Wife’s Life, Values, and Vision
Becky Lynch Drops Heel Character To Thank Fans Who Dressed Up As Her For Halloween

Justin Tv Justin: The Maverick Behind a Streaming Revolution
Chelsi McDonald WHDH Bio Wiki Age Parents Husband Salary and Net Worth

