Decoding HBr: The Simple Yet Powerful Lewis Structure Behind a Key Chemical Bond
Decoding HBr: The Simple Yet Powerful Lewis Structure Behind a Key Chemical Bond
In the world of chemical bonding, hydrogen bromide (HBr) stands out as a model compound that illustrates the bridge between acidic behavior and molecular geometry. Its Lewis structure reveals not only how electrons are arranged around atoms but also how simple molecular architecture underpins significant reactivity in industrial and biological processes. HBr, formed by bonding one hydrogen atom and one bromine atom via a polar covalent bond, offers a compact example of how electron distribution shapes chemistry in action.
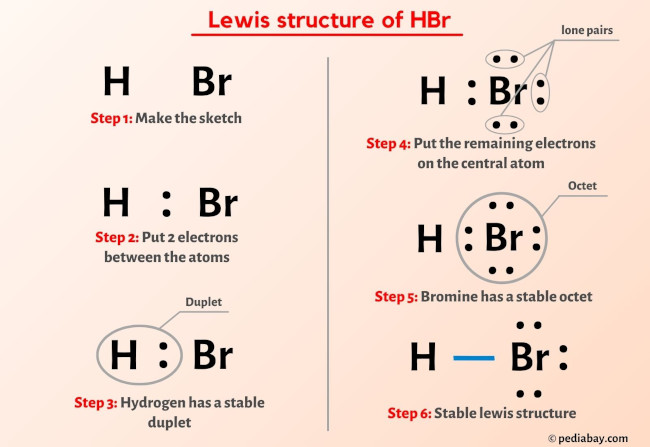
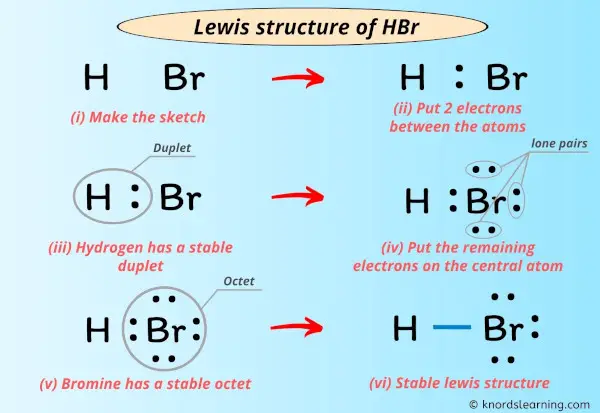
Hydrogen bromide consists of hydrogen (H) and bromine (Br) atoms connected through a shared electron pair, yet the structure carries critical asymmetry due to differing electronegativities. Using Lewis theory, scientists map out valence electrons to depict bonding and lone pairs with precision, unlocking insights into the molecule’s polarity, dipole moment, and chemistry. According to Purdue University’s Chemistry LibreTexts, “the hydrogen atom contributes one valence electron, while bromine—with seven—shares the remaining two to complete its octet,” forming a covalent bond with notable polarity.
The core Lewis structure of HBr features two key features: - A single covalent bond between H and Br, using two shared electrons. - A lone pair on bromine, remaining after bond formation, giving the molecule a trigonal planar electron geometry with a bent molecular shape. Despite its simplicity, this structure carries profound implications.
The electronegativity difference—hydrogen at 2.2 and bromine at 2.96—creates a dipole where bromine pulls electrons more strongly, resulting in partial negative charge (δ⁻) on bromine and partial positive charge (δ⁺) on hydrogen. This polarity makes HBr highly reactive, especially in proton transfer—fundamental to its role as a strong acid in aqueous solution. Another critical detail is bond length and energy.
Experimental data show the H–Br bond spans approximately 126 picometers, consistent with typical single covalent bonds but underscored by notable bond polarity. The molecule’s energy profile, derived from Lewis theory and supported by quantum mechanical calculations, confirms its stability under standard conditions while highlighting reactivity in the presence of nucleophiles or bases.
Chemical Behavior and Industrial Significance
HBr’s molecular form directly influences its utility across multiple domains.In industry, it serves as a precursor in the production of hydrobromic acid and organic bromides, critical in pharmaceuticals, flame retardants, and semiconductor manufacturing. As noted in a 2021 article by the American Chemical Society, “HBr’s dual nature as both a gas and a potent proton donor enables its use in selective organic transformations where precise reactivity is essential.” Beyond synthesis, HBr plays a role in atmospheric chemistry. When released into the air, it contributes to acid rain formation through reaction with water vapor to produce hydronium ions and bromide.
This environmental impact, rooted in molecular-level interactions, illustrates how basic structural understanding leads to broader ecosystem awareness. From a safety standpoint, HBr is highly corrosive and turbulent in concentrated forms, requiring careful handling. Its Lewis structure’s polar nature explains its hygroscopicity—readily absorbing moisture—which accelerates hydrolysis reactions, increasing hazard potential in storage and transport.
Chemists emphasize that molecular polarity dictates not only reactivity but also storage and safety protocols. Despite its hazards, such careful management underscores the value of core chemical insights. Knowing HBr’s structure allows engineers and researchers to anticipate behavior, design safer processes, and harness its reactivity predictably.
The Geometric Simplicity with Transformational Impact
At first glance, HBr presents a straightforward diatomic arrangement: one hydrogen and one bromine bonded covalently. Yet this simplicity masks the molecular’s geometric elegance. The Lewis structure reveals a planar electron domain with a 180-degree bond angle before lone pair repulsion reshapes the geometry into a bent configuration.This small deviation from linearity has big consequences, enhancing polarity and enabling diverse interactions. Such precision in structure informs behavior across phase transitions and thermodynamic conditions. Unlike symmetrical molecules that cancel dipoles, HBr’s bent shape ensures a net dipole moment—critical for solubility in polar solvents and interactions with charged surfaces.
In aqueous environments, for instance, HBr fully dissociates into H⁺ and Br⁻ ions, a behavior directly traceable to its initial electron distribution. “The bent structure of HBr amplifies electron density asymmetry, strengthening its proton-donating capacity,” explains Dr. Elena Marquez, physical chemist at Stanford University. “This structural feature makes it an ideal probe for studying Br–H bond cleavage and hydrogen bond formation—cornerstones of organic reaction mechanisms.”The role of electron geometry in reactivity
From Quantum Foundations to Real-World Applications
Behind every

Related Post

Louisville Football Injury Update: Get The Latest News on Player Status and Season Impact
Bring To Mind Nyt Science Stories That Blowed Your Mind—And Whether They Were Really Accurate

