Decoding Fluorine’s Chemistry: The Power of Its Lewis Dot Structure
Decoding Fluorine’s Chemistry: The Power of Its Lewis Dot Structure
Fluorine, the most electronegative element on the periodic table, plays a pivotal role in chemistry—from enabling vital biological functions to enabling industrial processes. At the heart of understanding fluorine’s reactivity and bonding behavior lies the Lewis dot structure, a foundational tool that reveals how electrons arrange around atoms. Beyond its deceptively simple appearance, the Lewis structure of fluorine and its compounds unlocks insights into molecular stability, polarity, and intermolecular forces—making it indispensable for students, researchers, and professionals alike.
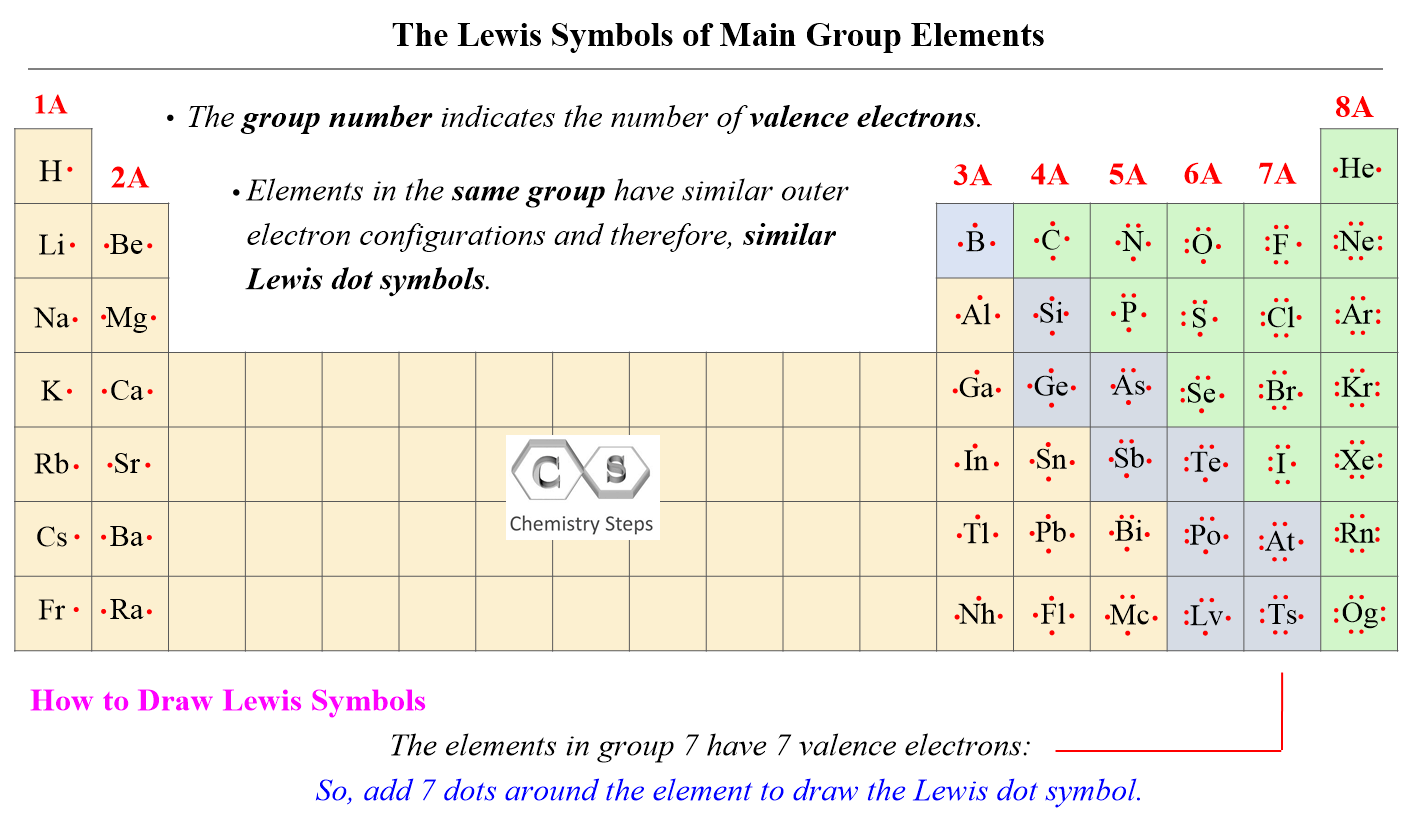
The Lewis dot structure for fluorine — F — reflects its electronic configuration: seven electrons total (heavenly stable with 7 ns² np⁵ configuration), always requiring one additional electron to complete a stable octet. Unlike many elements, fluorine typically appears as a monatomic gas F₂ in nature due to the exceptional strength of the F–F single bond. This bond forms a valence shell with just two electrons shared, yet the high electronegativity of fluorine creates an unusually strong covalent link, defined by strong orbital overlap and significant bond polarity.
“Fluorine’s bond is like a lightning strike—intense, precise, and uncompromising,” notes chemist Dr. Elena Torres from MIT’s Department of Chemical Sciences.
The Lewis structure itself, when simplified, shows a single shared pair between two fluorine atoms, emphasizing their symmetrical covalent bond. This representation, though elemental, carries profound implications: fluorine’s valence electrons are tightly held, limiting its tendency to lose electrons (it almost never acts as an oxidizing agent in the classical sense) but making it highly effective at attracting electrons.
More telling, the structure highlights why F₂ molecules resist decomposition and react selectively—such specificity drives fluorinated pharmaceuticals, surfactants, and refrigerants.
Electron Distribution: The Core of Fluorine’s Lewis Structure
Breaking down the Lewis electron layout for neutral fluorine reveals a critical insight: while fluorine gains a full valence shell through the octet rule, its atomic behavior remains distinct. With seven electrons total—six in p-orbitals and one in s-orbital—the element maintains seven valence electrons. In F₂, bonding occurs via sp³ hybridization overlap of minimally occupied orbitals, forming a single σ bond per molecule.
Each fluorine atom contributes one electron to this shared pair, resulting in a balanced covalent interaction, yet one electron short of a stable octet per atom.
The formal charge analysis underscores this balance: both fluorine atoms carry a formal charge of 0, with no lone pair excess beyond the shared bonding pair. This neutrality simplifies model predictions but contrasts with fluorine’s beiᯯied natural tendency to gain. Unlike alkali metals or main-group group elements, fluorine’s electron configuration makes electron loss energetically prohibitive—its high ionization energy of 1681 kJ/mol reinforces its reluctance to donate electrons.
Thus, Lewis structure for F → F₂ emphasizes a bond formed not by completion through ionic transfer, but by extreme electronegativity and orbital synergy.
Beyond the Gas: Lewis Structure Insights in Fluorine Compounds
Understanding the fluorine Lewis dot structure becomes even more powerful when applied to its key compounds—such as HF, CF₄, and NaF—where bonding environments differ dramatically. In hydrogen fluoride (HF), sulfur or carbon may bond to fluorine, but in most common forms, fluorine bonds exclusively to hydrogen via a polar covalent bond supported by the F–H dipole. Meanwhile, fluorine’s role in tetravolume carbon tetrafluoride (CF₄) involves sp³ hybridization with fully octet fluorine atoms, stabilized by strong second-shell interactions and minimal lone pair distortion.
In magnesium fluoride (MgF₂), a classic ionic compound, fluorine’s seven valence electrons complete Magnesium’s two available valence electrons via electron transfer, forming Mg²⁺ and F⁻ ions.
The Lewis representation—though simplified internally—reflects ionic charge balance, emphasizing how fluorine’s electronegativity shapes solid-state lattice energy and compound stability. This dichotomy—covalent mononuclear versus ionic multination—reveals the versatility of fluorine’s bonding, all rooted in its fundamental electron arrangement.
Practical Implications: Fluorine’s Lewis Structure in Science and Industry
Fluorine’s explanatory Lewis structure directly informs cutting-edge applications. In pharmaceutical chemistry, fluorinated compounds—such as fluorinated antibiotics and antivirals—exhibit enhanced metabolic stability and target affinity, largely due to fluorine’s electron-withdrawing effect and unique covalent bonding.
In materials science, fluoropolymers like PTFE (Teflon) owe their thermal and chemical resistance to fluorine’s omnipresent bonding, where strong F–C bonds resist degradation.
Industrial processes also rely on precise understanding: HF vapor is critical in semiconductor etching, where its polar covalent F–H dipole enables selective surface reactions. Similarly, in refrigerants like HFCs and HFOs, fluorine’s partial charge distribution and bond polarity tailor vapor pressure and thermodynamic efficiency. “Every engineered fluorine-based solution hinges on a deep grasp of how electrons arrange in target molecules,” says Dr.
Rajiv Mehta, a materials engineer at the National Fluorine Center. “The Lewis dot framework is the first bridge from theory to real-world application.”
The enduring value of Lewis dot structures lies in their ability to distill complex electronic behavior into intuitive blueprints. For fluorine, this means revealing not only how it bonds but why those bonds behave the way they do—powerful, precise, and purposeful.
Whether in nature’s simplest diatomic gas or in transformative industrial compounds, fluorine’s electron story remains one of chemistry’s most striking illustrations of structure dictating function.
As research advances into green fluorinations, sustainable fluorinated materials, and quantum chemical modeling, the Lewis structure remains a steadfast guide—proof that even the most elementary models, when understood deeply, unlock profound insights into nature’s chemistry.



Related Post

Film Action Terbaru 2023: Pembunuh Bayaran – Indonesia’s Gripping Crime Epic That Shakes Screens

When Money Talks Truth—Silence Speaks Louder: Unpacking the Proverb That Governs Wealth and Power
Mastering Geometric Dimensioning and Tolerance with the GD Benefits Portal: Unlocking Precision in T-Berial GD Data

