Boyle’s Law and Henry’s Law: The Hidden Forces Shaping Breath, Climate, and Industry
Boyle’s Law and Henry’s Law: The Hidden Forces Shaping Breath, Climate, and Industry
From the moment we inhale the first breath to the complex chemistry driving underwater exploration and industrial gas processing, two foundational gas laws—Boyle’s Law and Henry’s Law—play silent but pivotal roles. Together, these principles govern how pressure influences gas volume and how gases dissolve in liquids, influencing everything from lung physiology to carbon capture technologies. Understanding their interplay reveals not just the mechanics of breathing and oceanic gas exchange, but also the engineering behind innovations in medicine, energy, and environmental science.
Boyle’s Law: Pressure and Volume—The Breath Within
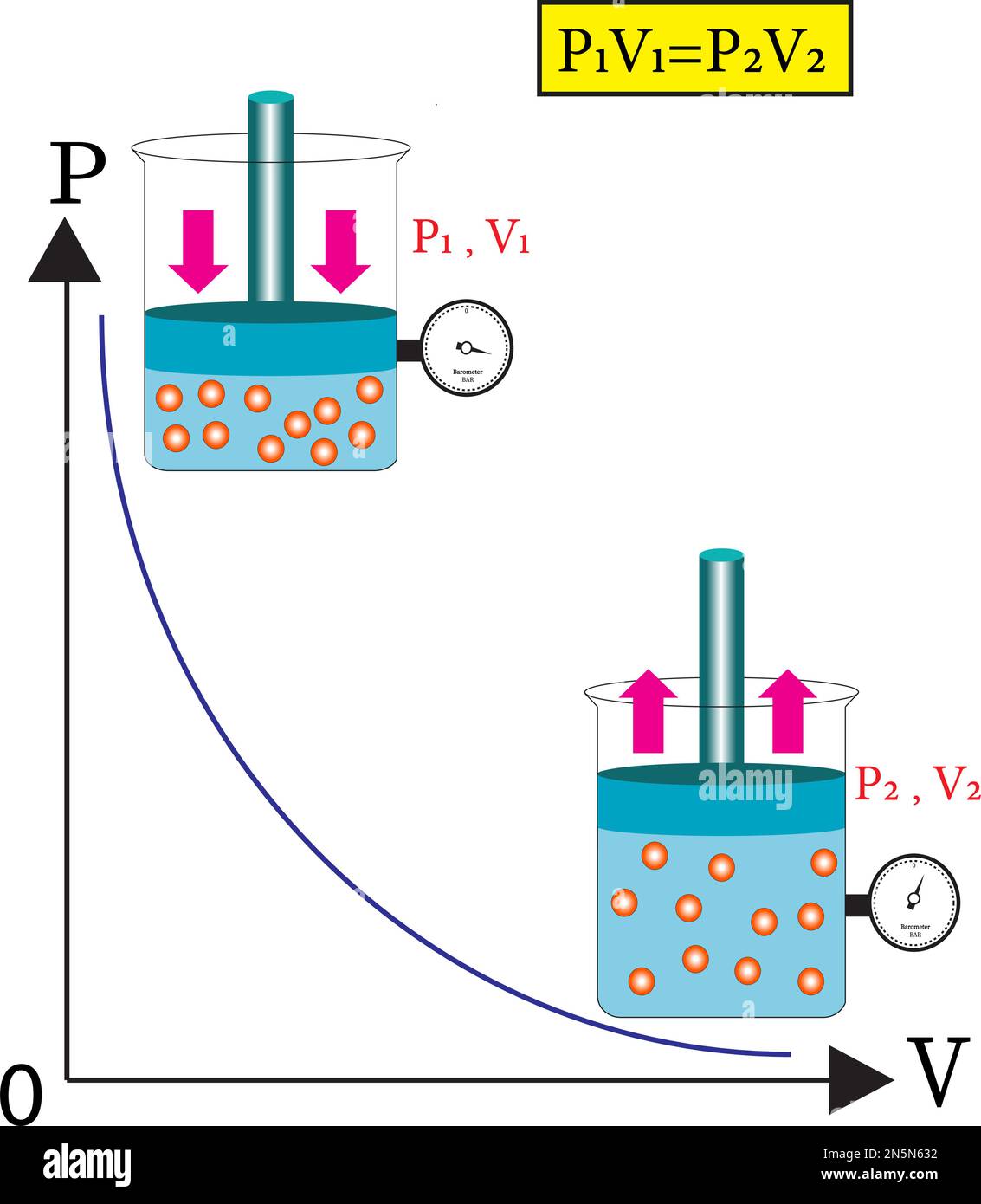
Boyle’s Law, first articulated by Robert Boyle in 1662, describes a simple yet profound relationship: at constant temperature, the volume of a gas is inversely proportional to its pressure. Formalized as \( P \propto \frac{1}{V} \), or more precisely \( P_1V_1 = P_2V_2 \), the law reveals a direct tension between force and space. As pressure increases, gas molecules are squeezed closer together, reducing volume; as pressure decreases, molecules expand, increasing volume.This principle is most evident in human respiration. During inhalation, the diaphragm contracts and expands the thoracic cavity, lowering internal pressure within the lungs relative to atmospheric pressure—approximately 1 kPa difference drives air into the lungs. The inverse relationship becomes apparent in how breathing adjusts: increased exhalation raises lung volume and pressure, pushing air out as Boyle’s equation dictates.
Beyond breathing, Boyle’s Law underpins a range of practical applications. In pneumatic tools—air-powered drills or impact wrenches—pressure regulation depends on controlled volume changes. Similarly, scuba divers must factor in Boyle’s Law when descending: rising pressure compresses air in breathing tanks and the body’s lungs, increasing internal energy and risk of injury if not managed.
The law’s simplicity belies its power: it explains why compressed gas ignites risk in industrial settings and why altitude profoundly affects oxygen delivery. At higher elevations, atmospheric pressure drops, expanding gas volumes in breathing apparatus and lungs—requiring enriched oxygen supplies to maintain pressure balance and preserve physiology.
Henry’s Law: The Solubility of Gases—When Liquids Breathe In
While Boyle’s Law governs gas volume under pressure, Henry’s Law governs gas solubility in liquids—a law originally formulated by William Henry in 1803.It states that a gas dissolved in a liquid reaches equilibrium when its partial pressure above the liquid equals the product of the gas’s concentration in the liquid and Henry’s constant (\( C = k_H \cdot P \)). In simpler terms: the more pressure a gas experiences above a liquid, the more of it dissolves. This principle governs critical systems from oceanic carbon sinks to medical anesthesia.
In the world’s oceans, Henry’s Law explains how atmospheric carbon dioxide dissolves into surface waters, playing a vital role in the global carbon cycle. Yet surplus CO₂ absorption contributes to ocean acidification, dissolving shell-forming minerals and threatening marine ecosystems. In medicine, Henry’s Law enables safe anesthetic delivery.
Nitrous oxide, commonly used in surgical sedation, dissolves efficiently under controlled pressure in a patient’s bloodstream, then releases as pressure eases—allowing rapid drying of the lungs post-procedure. This precise solubility balance ensures effective dosing without toxic buildup. Henry’s constant varies with temperature and gas type: colder water holds more dissolved gas, which is why marine creatures face risks in warming oceans—warmer temperatures reduce dissolved oxygen, stressing fish and invertebrates alike.
Likewise, pressurized scuba tanks depend on Henry’s Law—gas density increases under pressure, enabling divers to carry sufficient oxygen for deep dives.
The Synergy of Pressure and Solubility in Extreme Environments
The convergence of Boyle’s and Henry’s Laws becomes most pronounced in extreme environments—deep-sea pressure zones and high-altitude habitats—where pressure and temperature extremes redefine gas behavior. At abyssal depths, pressures exceed 100 atmospheres.Here, Boyle’s Law dictates a near-immediacy to gas expansion: even slow ascents compress bubbles and dissolved gases, risking decompression sickness (“the bends”) when pressure drops faster than solubility can release gases. Henry’s Law amplifies this danger: dissolved gases in blood exceed saturation levels, forming bubbles upon rapid decompression. Modern diving protocols, using staged pressure chambers and controlled ascent, counteract both laws’ effects—slowing volume expansion via gradual pressure reduction and maintaining safe gas solubility through oxygen-enriched mixes.
Conversely, at high altitudes, reduced atmospheric pressure diminishes Boyle’s effect—lungs expand more freely, but inhaled oxygen volume per breath decreases. Simultaneously, Henry’s Law dictates diminished physical gas uptake: lower air pressure cuts nitrogen and oxygen solubility in blood, increasing altitude sickness risk. Innovations like supplemental oxygen systems and pressurized aircraft cabins leverage knowledge of both laws to maintain safe internal pressures and gas equilibria.
Beyond physiology, industrial engineering draws heavily from these laws. In hyperbaric chambers, elevated pressures increase oxygen solubility in blood—enhancing healing, but requiring precise pressure control to avoid toxic dissolved nitrogen. In carbon capture and storage, Henry’s Law guides the injection of CO₂ into deep geological formations, where pressure keeps it dissolved, preventing atmosphere contamination.
Boyle’s and Henry’s laws, though centuries old, remain indispensable in decoding gas behavior across scales—from human respiration to planetary climate. Their elegant simplicity reveals deep truths: pressure governs volume, solubility governs exchange. Mastery of these principles enables safer medicine, cleaner energy, and deeper oceanic exploration.
In every breath drawn and every sensor reading, these laws breathe life into science.


Related Post
Unpacking the 'Fun With Family Fun Pack': A Deep Dive into Modern Entertainment Bundles

P Diddy’s Mother: The Modest Mind Behind the Music Mogul’s Legacy

Unraveling the Truth Behind Jonny Harris' Illness: A Public Figure’s Struggle Revealed
Marisa Woloszyn TMJ4 Bio Wiki Age Height Family Husband Salary And Net Worth

