Aluminum’s Atomic Blueprint: Decoding Its Structure and Properties Through Lewis Dot Formulas
Aluminum’s Atomic Blueprint: Decoding Its Structure and Properties Through Lewis Dot Formulas
Aluminum, the third most abundant element on Earth and a linchpin of modern industry, owes its remarkable versatility to its atomic arrangement—revealed clearly through Lewis dot structure analysis. This fundamental representation of valence electrons not only explains aluminum’s chemical reactivity but also underpins its stability, conductivity, and wide-ranging applications in everything from transportation to electronics. By examining aluminum’s Lewis dot structure, we uncover a material engineered for strength and adaptability at the atomic level, a quiet architect behind countless innovations.
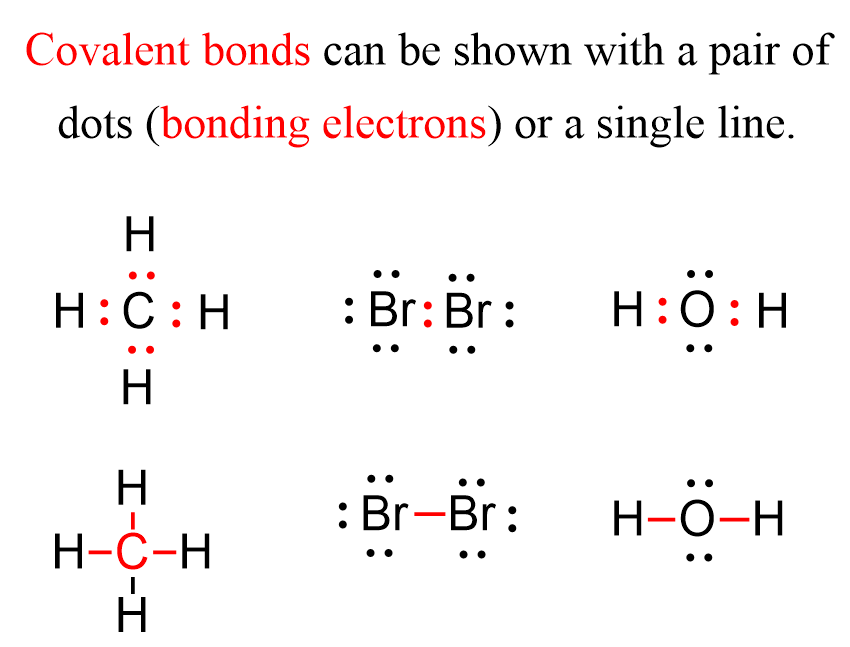
Lewis dot structures offer a precise visual language for understanding an atom’s electron disposition—particularly the valence electrons critical to bonding.
For aluminum (Al), with an atomic number of 13, the neutral atom possesses three valence electrons, positioned in the outermost shell following the Aufbau principle. These electrons—denoted here—form the foundation of aluminum’s chemical behavior, enabling it to readily share or transfer electrons to achieve stability. Unlike heavier metals with more complex d-orbital interactions, aluminum’s chemistry is straightforward, yet profoundly influential.
Unveiling Aluminum’s Electron Equilibrium: The Lewis Dot Formula
To construct aluminum’s Lewis dot structure, begin with the atomic symbol Al, followed by three dots arranged around it to represent its three valence electrons.
Unlike carbon or oxygen, aluminum does not participate in double bonding under standard conditions; instead, it forms stable single bonds by sharing electrons. The full configuration is represented as:
··Al:··
This concise symbolization captures not just electron count, but the essence of aluminum’s bonding nature—simple, efficient, and highly directional. Each dot signifies a separate valence electron contributing to the atom’s pursuit of a noble gas configuration, a classical drive toward stability.
Though aluminum lacks expanded octets (unlike sulfur or phosphorus), its dot structure reveals a preference for spin pairing and localized bonding, consistent with typical group 13 elements.
In a Lewis structure, aluminum exists as a monoatomic species, though in alloys and compounds, these dots interact dynamically. In its purest form, the structure supports metallic bonding: delocalized valence electrons free to move across a lattice, giving aluminum its signature conductivity and malleability. Yet the dots themselves remain key—anchoring reactivity and guiding design in industrial chemistry.
The Role of Valence Electrons in Aluminum’s Reactivity
Aluminum’s three valence electrons define its chemical personality.
In isolation, the atom tends to lose these electrons to achieve a noble gas configuration (neon-like stability), forming Al³⁺ ions. But in practice, aluminum readily shares electrons to form covalent or ionic bonds—depending on the partner. For example, in aluminum chloride (AlCl₃), the Lewis structure shows aluminum sharing electrons with three chlorine atoms:
- • A central Al³⁺ surrounded by three Cl⁻ ions in an ionic crystal lattice.
- • In organic halides like AlCl₃, aluminum engages in covalent networking, with Lewis dots symbolizing shared pairs.
This electron-sharing behavior is central to aluminum’s utility in synthesis, catalysis, and material science.
Unlike metals that rely solely on metallic bonding, aluminum’s dual bonding modes—ionic and covalent—enhance its adaptability. The Lewis dot structure, therefore, is not mere representation but a predictive tool, illuminating reaction pathways and compound stability.
Metallic Bonding: A Network of Electrons and Ions
Beyond discrete compounds, aluminum’s bulk form exemplifies metallic bonding—a phenomenon their Lewis dot structure helps explain. Although Al atoms are represented individually, their valence electrons are effectively delocalized across a network of positive ion cores.
This “sea” of electrons enables heat and electrical conductivity,



Related Post
Bret Hart Says Vince McMahon Allegations Are Not The Only Incident of Predatory Behavior

Exploring The Family Life of Carrie Preston: Secrets and Strength in Raising Her Children
Shari Headley Family Untold Stories and Personal Insights: A Window into Resilience, Legacy, and Quiet Strength

